Summary
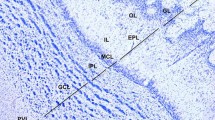
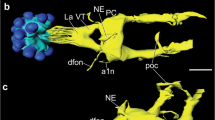
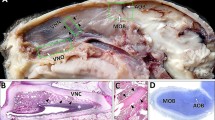
The distribution of dopamine (DA) and the biosynthetic enzyme tyrosine hydroxylase (TH) has been studied immunohistochemically in the brain of the adult South African clawed frog, Xenopus laevis. The goals of the present study are, firstly, to provide detailed information on the DA system of the brain of a species which is commonly used in laboratories as an experimental model and, secondly, to enhance our insight into primitive and derived characters of this catecholaminergic system in amphibians. Dopamine-immunoreactive cell bodies are present in the olfactory bulb, the preoptic area, the suprachiasmatic nucleus, the nucleus of the periventricular organ and its accompanying cells, the nucleus of the posterior tubercle, the posterior thalamic nucleus, the midbrain tegmentum, around the solitary tract, in the ependymal layer along the midline of the caudal rhombencephalon, and along the central canal of the spinal cord. In contrast to the DA antiserum, the TH antiserum fails to stain the liquor-contacting cells in the periventricular organ. On the contrary, the latter antiserum reveals additional immunoreactive cell bodies in the olfactory bulb, the isthmic region and the caudal brainstem. Both antisera yield an almost identical distribution of fibers. Distinct fiber plexuses are observed in the olfactory bulb, the basal forebrain, the hypothalamus and the intermediate lobe of the hypophysis. Features that Xenopus shares with other anurans are the larger number of DAi cells, which are generally smaller in size than those observed in urodeles, and the lack of DAi fibers in pallial structures. On the other hand, the paired midbrain DA cell group and the innervation of the tectum of Xenopus resemble those found in the newt rather than those in frogs. Despite the existence of these species differences, the brain of Xenopus offers an excellent model for studying general aspects of neurotransmitter interactions and the development of catecholamine systems in this class of vertebrates.
Similar content being viewed by others
References
Brauth SE (1988) Catecholamine neurons in the brainstem of the reptile Caiman crocodilus. J Comp Neurol 270:313–326
de Rijk EPCT (1991) Neural control of melanotrope cell activity in Xenopus laevis. Ph.D. Thesis, Nijmegen
de Rijk EPCT, van Strien FJC, Roubos EW (1992) Demonstration of coexisting catecholamine (dopamine), amino acid (GABA), and peptide (NPY) involved in inhibition of melanotrope cell activity in Xenopus laevis: a quantitative ultrastructural, freeze-substitution immunocytochemical study. J Neurosci 12:864–871
González A (1992) Immunohistochemical demonstration of catecholamine systems in the brain of amphibians. Abstr 7th Int Catechol Symp, Amsterdam, p 117
González A, Smeets WJAJ (1991) Comparative analysis of dopamine and tyrosine hydroxylase immunoreactivities in the brain of two amphibians, the anuran Rana ridibunda and the urodele Pleurodeles waltlii. J Comp Neurol 303:457–477
González A, Smeets WJAJ (1992a) Comparative analysis of the vasotocinergic and mesotocinergic cells and fibers in the brain of two amphibians, the anuran Rana ridibunda and the urodele Pleurodeles waltlii. J Comp Neurol 315:53–73
González A, Smeets WJAJ (1992b) Distribution of vasotocin- and mesotocin-like immunoreactivities in the brain of the South African clawed frog Xenopus laevis. J Chem Neuroanat 5:465–479
Haber SN, Groenewegen HJ (1989) The interrelationship of the distribution of neuropeptides and tyrosine hydroxylase immunoreactivity in the human substantia nigra. J Comp Neurol 290:53–68
Linden van der JAM, ten Donkelaar HJ, de Boer-van Huizen R (1990) Development of olivocerebellar fibers in the clawed toad, Xenopus laevis: a light and electron microscopical HRP study. J Comp Neurol 293:236–252
Meredith GE, Smeets WJAJ (1987) Immunocytochemical analysis of the dopamine system in the forebrain and midbrain of Raja radiata: evidence for a substantia nigra and ventral tegmental area in cartilaginous fish. J Comp Neurol 265:530–548
Mier P van, ten Donkelaar HJ (1984) Early development of descending pathways from the brain stem to the spinal cord in Xenopus laevis. Anat Embryol 170:295–306
Mier P van, Joosten HWJ, van Rheden R, ten Donkelaar HJ (1986) The development of serotoninergic raphespinal projections in Xenopus laevis. Int J Neurosci 4:465–475
Mier P van, van Rheden R, ten Donkelaar HJ (1985) The development of the dendritic organization of primary and secondary motoneurons in the spinal cord of Xenopus laevis. Anat Embryol 172:311–324
Nakai Y, Ochiai H, Shioda S (1977) Cytological evidence for different types of cerebrospinal fluid-contacting subependymal cells in the preoptic and infundibular recesses of the frog Cell Tissue Res 176:317–334
Nieuwkoop PD, Faber J (1967) Normal table of Xenopus laevis (Daudin), North-Holland, Amsterdam
Northcutt RG, Reiner A, Karten HJ (1988) An immunohistochemical study of the telencephalon of the spiny dogfish, Squalus acanthias. J Comp Neurol 277:250–267
Roubos EW (1992) Routing and release of input and output messengers of peptidergic systems. Prog Brain Res 92:257–265
Smeets WJAJ (1988) Distribution of dopamine immunoreactivity in the forebrain and midbrain of the snake Python regius: a study with antibodies against dopamine. J Comp Neurol 271:115–129
Smeets WJAJ, González A (1990) Are putative dopamine-accumulating cell bodies in the hypothalamic periventricular organ a primitive brain character of non-mammalian vertebrates? Neurosci Lett 114:248–252
Smeets WJAJ, Steinbusch HWM (1990) New insights into the reptilian catecholaminergic systems as revealed by antibodies against the neurotransmitters and their synthetic enzymes. J Chem Neuroanat 3:25–43
Smeets WJAJ, Hoogland PV, Voorn P (1986) Distribution of dopamine immunoreactivity in the forebrain and midbrain of the lizard Gekko gecko: an immunohistochemical study with antibodies against dopamine. J Comp Neurol 253:46–60
Smeets WJAJ, Jonker AJ, Hoogland PV (1987) Distribution of dopamine in the forebrain and midbrain of the red-eared turtle, Pseudemys scripta elegans, reinvestigated using antibodies against dopamine. Brain Behav Evol 30:121–142
Smeets WJAJ, Kidjan MN, Jonker AJ (1991) α-MPT does not affect dopamine levels in the periventricular organ of lizards. Neuroreport 2:369–372
Stuesse SL, Cruce WLR (1992) Distribution of tyrosine hydroxylase, serotonin, and leu-enkephalin immunoreactive cells in the brainstem of a shark, Squalus acanthias. Brain Behav Evol 39:77–92
Stuesse SL, Cruce WLR, Northcutt RG (1990) Distribution of tyrosine hydroxylase- and serotonin-immunoreactive cells in the central nervous system of the thornback guitarfish, Platyrhinoidis triseriata. J Chem Neuroanat 3:45–58
Stuesse SL, Cruce WLR, Northcutt RG (1991) Localization of serotonin, tyrosine hydroxylase, and leu-enkephalin immunoreactive cells in the brainstem of the horn shark, Heterodontus francisci. J Comp Neurol 308:277–292
Tay D, Straznicky C (1982) The development of the diencephalon in Xenopus: an autoradiographic study. Anat Embryol 163:371–388
Terlou M, Ploemacher RE (1973) The distribution of monoamines in the tel-, di- and mesencephalon of Xenopus laevis tadpoles, with special reference to the hypothalamo-hypophyseal system. Z Zellforsch Mikrosk Anat 137:521–540
Thors F, de Kort EJM, Nieuwenhuys R (1982) On the development of the spinal cord of the clawed frog, Xenopus laevis. II. Experimental analysis of differentiation and migration. Anat Embryol 164:443–454
Tonon MC, Bosler O, Stoeckel ME, Pelletier G, Tappaz M, Vaudry H (1992) Co-localization of tyrosine hydroxylase, GABA and neuropeptide Y within axon terminals innervating the intermediate lobe of the frog Rana ridibunda. J Comp Neurol 319:599–605
Tuinhof R, de Rijk EPCT, van Strien FJC, Wismans RGP, Smeets WJAJ, Roubos EW (1992) Role of coexisting dopamine, GABA and NPY in the control of background adaptation of the amphibian Xenopus laevis. Abstr 7th Intern Catechol Symp, Amsterdam, p 319
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
González, A., Tuinhof, R. & Smeets, W.J.A.J. Distribution of tyrosine hydroxylase and dopamine immunoreactivities in the brain of the South African clawed frog Xenopus laevis . Anat Embryol 187, 193–201 (1993). https://doi.org/10.1007/BF00171750
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00171750