Summary
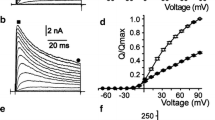
To study the mechanism of use dependence, we investigated the influence of 0.5 tocainide on the amplitude of sodium currents elicited by membrane depolarizations with standard test pulses in voltage-clamped human myoballs. For comparison, the experiments were also conducted with 1 mM benzocaine, a drug with almost no use dependence. These concentrations were so chosen that without stimulation and at a holding potential of −135 mV, either drug blocked about 50% of the channels (tonic block). With repetitive stimulation at 1 Hz, tocainide blocked about 75% of the channels that had remained open in the rested state (phasic block), while benzocaine had little such effect. The potential dependence of steady-state inactivation (h∞ curves) of the sodium channels in these myoballs depended on the duration of the prepotential indicating that they possess at least two states of inactivation: fast and intermediate. The two drugs differed in their effects on these two states. Benzocaine always produced a left-shift of the h∞ curve, no matter whether the duration of the conditioning pulse was short (8 ms) or long (512 ms) indicating that it can bind when the channel is in the state of fast inactivation. Tocainide shifted the h∞ curve only with long prepulses, i.e. when the sodium channels were in the state of intermediate inactivation. The recovery from inactivation, a process governed by two time constants in the absence of drugs, was also differently influenced by the two drugs. In the presence of tocainide, the channels mainly recovered with the slow time constant and this time constant was significantly increased, whereas benzocaine did not substantially modify this biphasic process. The results are in agreement with the hypothesis that drug binding depends on the state of the channel. The different use dependences of tocainide and benzocaine are explained by the fact that they bind favourably to the sodium channels when they are in the states of intermediate and fast inactivation, respectively.
Similar content being viewed by others
References
Bezanilla F, Armstrong CM (1977) Inactivation of the sodium channel. I. Sodium current experiments. J Gen Physiol 70:549–566
Carmeliet E (1987) Slow inactivation of the sodium current in rabbit cardiac Purkinje fibres. Pflügers Arch 408:18–26
Carmeliet E (1988) Activation block and trapping of penticainide, a disopyramide analogue, in the Na+ channel of rabbit cardiac Purkinje fibers. Circ Res 63:50–60
Chiu SY (1977) Inactivation of sodium channels: second order kinetics in myelinated nerve. J Physiol (Lond) 273:573–596
DeLuca A, Brinkmeier H, Fakler B, Pröbstle T, Rüdel R (1990) Local anaesthetic drugs with different use dependence have different effects on sodium channel inactivation. Pflügers Arch 415:R8
Elliott JR, Haydon DA, Hendry BM (1987) The mechanisms of sodium current inhibition by benzocaine in the squid giant axon. Pflügers Arch 409:596–600
Fakler B, Ruppersberg JP, Spittelmeister W, Rüdel R (1990) Inactivation of human sodium channels and the effect of tocainide. Pflügers Arch 415:693–700
Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch clamp technique for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391:85–100
Hille B (1977) Local anesthetics: hydrophilic and hydrophobic pathway for the drug receptor reaction. J Gen Physiol 69:497–515
Hodgkin AL, Huxley AF (1952) A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol (Lond) 117:500–544
Hondeghem LM, Katzung BG (1977) Time and voltage-dependent interactions of antiarhythmic drugs with cardiac sodium channels. Biochim Biophys Acta 472:373–398
Huang L-YM, Ehrenstein G (1981) Local anesthetics QX572 and benzocaine act at separate sites on the batrachotoxin-activated sodium channel. J Gen Physiol 77:137–153
Luther MA, Schoepfer R, Whiting P, Casey B, Blatt Y, Montal MS, Montal M, Lindstrom J (1989) A muscle acetylcholine receptor is expressed in the human cerebellar modulloblastoma cell line TE671. J Neurosci 9:1082–1096
Mrose HE, Ritchie JM (1978) Local anesthetics: do benzocaine and lidocaine act at the same site? J Gen Physiol 71:223–225
Pröbstle T, Rüdel R, Ruppersberg JP (1988) Hodgkin-Huxley parameters of the sodium channels in human myoballs. Pflügers Arch 412:264–269
Ricker K, Haass A, Rüdel R, Böhlen R, Mertens HG (1980) Successful treatment of paramyotonia congenita (Eulenburg): muscle stiffness and weakness prevented by tocainide. J Neurol Neurosurg Psychiatry 43:268–271
Rüdel R, Dengler R, Ricker K, Haass A, Emser W (1980) Improved therapy of myotonia with the lidocaine derivative tocainide. J Neurol 222:275–278
Rüdel R, Fakler B (1991) Ultra-slow, slow, intermediate and fast inactivation of human sodium channels. In: Simmons R (ed) Muscular contraction. Cambridge University Press, Cambridge (in press)
Ruff RL, Simoncini L, StiIhmer W (1988) Slow sodium channel inactivation in mammalian muscle: a possible role in regulating excitability. Muscle Nerve 11:502–510
Schmidtmayer J, Ulbricht W (1980) Interaction of lidocaine and benzocaine in blocking sodium channels. Pflügers Arch 387:47–54
Schwarz W, Palade PT, Hille B (1977) Local anesthetics: effect of pH on use-dependent block of sodium channels in frog muscle. Biophys J 20:343–368
Stratton MR, Reeves BR, Cooper CS (1989) Misidentified cell. Nature 337:311–312
Starmer CF, Grant AO, Strauss HC (1984) Mechanisms of use-dependent block of sodium channels in excitable membranes by local anesthetics. Biophys J 46:15–27
Tricarico D, Fakler B, Spittelmeister W, Ruppersberg JP, Sttitzel R, Franchini C, Tortorella V, Conte-Camerino D, Rüdel R (1991) Stereoselective interaction of tocainide and its chiral analogs with the sodium channels in human myoballs. Pflügers Arch 418: 234–237
Vaughan Williams EM (1984) A classification of antiarrhythmic actions reassessed after a decade of new drugs. J Clin Pharmacol 24: 129–147
Wang GK, Brodwick MG, Eaton DC, Strichartz GR (1987) Inhibition of sodium current by local anesthetics in chloramine T treated squid axons; the role of channel activation. J Gen Physiol 89:645–667
Author information
Authors and Affiliations
Additional information
Send offprint requests to R. Rüdel at the above address
Rights and permissions
About this article
Cite this article
DeLuca, A., Pröbstle, T., Brinkmeier, H. et al. The different use dependences of tocainide and benzocaine are correlated with different effects on sodium channel inactivation. Naunyn-Schmiedeberg's Arch Pharmacol 344, 596–601 (1991). https://doi.org/10.1007/BF00170658
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00170658