Summary
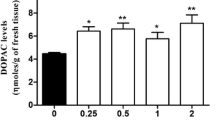
The biochemical, endocrine, receptor binding, and behavioral effects of the putative dopamine autoreceptor agonist, U-86170F, were evaluated in various in vivo and in vitro models. U-86170F and apomorphine were shown to cause a significant reversal of the effects of γ-butyrolactone (GBL) on dopamine accumulation in mouse striata. In contrast to apomorphine, U-86170F had a ceiling effect on the extent of the reversal of GBL effects (55%), whereas apomorphine had an 82% reversal. The effect on striatal homovanillic acid (HVA) levels was also monitored, and both compounds exerted a similar and significant reduction in striatal HVA. A comparison was made between the effects of intraperitoneal (i.p.) and oral administration of U-86170F in the α-methyl-p-tyrosine (α-MPT)/prolactin model in rats. When administered by the i.p. route, U-86170F suppressed the effects of α-MPT on prolactin level increase, having an ED50 of about 0.03 mg/kg, and when administered by the oral route, its ED50 was approximately 0.1 mg/kg. U-86170F has been shown to be a potent dopamine autoreceptor agonist in the GBL, prolactin, and HVA models, with an effective i.p. dose of approximately 0.03 mg/kg. When evaluated for postsynaptic dopaminergic activity in the reserpinized mouse model, and compared to apomorphine, U-86170F was found to increase locomotor activity, but its maximum effect was only 65% of that attained with apomorphine. Higher doses were needed for postsynaptic effects.
In receptor binding studies using cloned D2 receptor preparations, U-86170F was found to exhibit agonist binding properties similar to dopamine as demonstrated by their inhibition of 3H-raclopride binding. Both compounds exhibited biphasic inhibition curves, with U-86170F having Ki values of 7.5 nM and 250 nM, and for dopamine the Ki values were 34.7 nM and 1031 nM. Binding studies conducted in the presence of GTP yielded only one site with Kis of 289 nM and 670 nM, for both U-86170F and dopamine, respectively.
The results presented in this report demonstrated that U-86170F is a potent dopamine autoreceptor agonist, with limited activity at the postsynaptic receptor.
Similar content being viewed by others
References
Billard W Ruperto V, Crosby G, Lorio LC, Barnett A (1984) Characterization of binding of 3H-SCH-23390, a selective D1 receptor antagonist ligand in rat striatum. Life Sci 35:1885–1893
Carlsson A (1978) Antipsychotic drugs, neurotransmitters, and schizophrenia. Am J Psychiatry 135:164–173
Carlsson A (1975) Receptor-mediated control of dopamine metabolism. In: Usdin E, Bunney WE (eds) Pre- and postsynaptic receptors. Marcel Dekker, New York, pp 49–63
Chio LC, Hess GF, Graham RS, Huff RM (1990) A second molecular form of D2 dopamine receptor in rat and bovine caudate nucleus. Nature 343:266–269
Donoso AD, Bishop W Fawcett CP, Krulich L, McCann SM (1970) Effect of drugs that modify brain monoamine concentrations on plasma gonadotropin and prolactin levels in the rat. Endocrinology 89:774–784
Gianutsos G, Thornberg JE, Moore KE (1976) Differential actions of dopamine agonists and antagonists on the γ-butyrolactone-induced increase in mouse brain dopamine. Psychopharmacology 50:225–229
Golzan H, Mestikawy SEI, Pichat L, Glowinski J, Hamon M (1983) Identification of presynaptic serotonin autoreceptors using a new ligand: 3H-DAT. Nature 140–142
Hjorth S, Carlsson A, Clark D, Svensson K, Wikstrom H, Sanchez D, Lindberg P, Hacksell U, Arvidsson LE, Johansson A, Nilsson JLG (1983) Central dopamine agonist and antagonist actions of the enantiomers of 3-PPP. Psychopharmacology 81:89–99
Hjorth S, Svensson K, Carlsson A, Wikstrom H, Andersson B (1986) Central dopaminergic properties of HW 165 and its enantiomers; Transoctahydrobenzo(f)quinoline congeners of 3-PPP. Naunyn-Schmiedeberg's Arch Pharmacol 333:205–218
Kohler C, Hall H, Ogren S-V, Gawell G (1985) Specific in vitro and in vivo binding of 3H-raclopride. Biochem Pharmacol 2251–2259
Meller E, Helmet-Matyjek R, Bohmaker K, Adler CH, Friedhoff AJ, Goldstein M (1986) Receptor reserve at striatal dopamine autoreceptors: implications for selectivity of dopamine agonists. Eur J Pharmacol 123:311–314
Roth RH (1979) Dopamine autoreceptors: pharmacology, function and comparison with post-synaptic receptors. Commun Psychopharmacol 3:429–445
Sokoloff P, Giros B, Mattes M-P, Bouthenet M-L, Schwartz J-C (1990) Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature 347:146–151
Tamminga CA, Gotta MD, Thaker GK, Alphs LD, Foster NL (1986) Dopamine agonist treatment of schizophrenia with N-propylnorapomorphine. Arch Gen Psychiatry 43:398–402
Von Voigtlander PF, Althaus JS, Comacho Ochoa M, Neff G (1989) Dopamine receptor agonist activity of U-66444B and its enantiomers. Drug Developm Res 17:71–81
Walters JR, Bergstrom DA, Carlson JH, Chase TN, Braun AR (1987) D1 dopamine receptor activation required for postsynaptic expression of D2 agonist effects. Science 236:719–722
Author information
Authors and Affiliations
Additional information
Send offprint requests to R.A. Lahti at the above address
Rights and permissions
About this article
Cite this article
Lahti, R.A., Evans, D.L., Figur, L.M. et al. Pre- and postsynaptic dopaminergic activities of U-86170F. Naunyn-Schmiedeberg's Arch Pharmacol 344, 509–513 (1991). https://doi.org/10.1007/BF00170644
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00170644