Summary
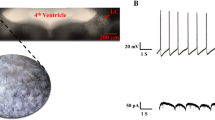
In pontine slices of the rat brain, the frequency of spontaneous action potentials of locus coeruleus (LC) neurones was recorded extracellularly. Noradrenaline 0.1–100 μmol/l, UK 14,304 0.01–100 nmol/l, [Met5]-enkephalin 1–10,000 nmol/l and [D-Ala2, D-Leu5]enkephalin 0.1–1,000 nmol/l, all depressed the firing rate. Rauwolscine 1 μmol/l antagonized the effects of both noradrenaline and UK 14,304, but potentiated the effects of [Met']enkephalin and [D-Ala2, D-Leu5]enkephalin. Idazoxan 1 μmol/l acted in a similar manner. Prazosin 1 μmol/l did not change the effects of either noradrenaline or [Met5]enkephalin. Naloxone 0.1 μmol/l antagonized both [Met']enkephalin and [D-Ala2, D-Leu5]enkephalin, but failed to alter the effects of either noradrenaline or UK 14,304. Rauwolscine, idazoxan and prazosin, all 1 μmol/l, as well as naloxone 0.1 μmol/l, did not influence the firing rate when given alone. Desipramine 1 μmol/l inhibited the discharge of action potentials in a rauwolscine-antagonizable manner. Noradrenaline 10 μmol/l produced the same depression of firing, both in the presence of noradrenaline 1 μmol/l and [Met5]enkephalin 0.03 μmol/l. Likewise, the effect of [Met5]enkephalin 0.3 μmol/l was the same, irrespective of whether it was added to a medium containing [Met5]enkephalin 0.03 μmol/l or noradrenaline 1 μmol/l. The spontaneous activity of LC neurones is inhibited by somatic α2-adrenoceptors and opioid μ-receptors. We suggest that the two receptors interact with each other at a site located between themselves and not in the subsequent common signal transduction system.
Similar content being viewed by others
References
Aghajanian GK, VanderMaelen CP (1982) α2-Adrenoceptor-mediated hyperpolarization of locus coerulcus neurones: intracellular studies in vivo. Science 215:1394–1396
Aghajanian GK, Wang YY (1986) Pertussis toxin blocks the outward currents evoked by opiate and α2-agonists in locus coeruleus neurons. Brain Res 371:390–394
Aghajanian GK, Wang YY (1987) Common α2- and opiate effector mechanisms in the locus coeruleus: intracellular studies in brain slices. Neuropharmacology 26:793–799
Allgaier C, Daschmann B, Sieverling J, Hertting G (1989) Presynaptic κ-opioid receptors on noradrenergic nerve terminals couple to G proteins and interact with the α2-adrenoceptors. J Neurochem 53:1629–1635
Cederbaum JM, Aghajanian GK (1977) Catecholamine receptors of locus coeruleus neurones: pharmacological characterization. Eur J Pharmacol 44:375–385
Doxey JC, Smith CFC, Walker JM (1977) Selectivity of blocking agents for pre- and postsynaptic α-adrenoceptors. Br J Pharmacol 60:91–96
Doxey JC, Roach AG, Smith CFC (1983) Studies on RX 781094: a selective, potent and specific antagonist of α2-adrenoceptors. Br J Pharmacol 78:489–505
Doxey JC, Lane AC, Roach AG, Smith CFC, Walter DS (1985) Selective α2-adrenoceptor agonists and antagonists. In: Szabadi E, Bradshaw CM, Nahorski SR (eds) Pharmacology of adrenoceptors. VCH, Weinheim, pp 13–22
Egan TM, Henderson G, North RA, Williams JT (1983) Noradrenaline-mediated synaptic inhibition in rat locus coeruleus neurones. J Physiol 345:477–488
Foote SL, Aston-Jones G, Bloom FE (1980) Impulse activity of locus coeruleus neurones in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci USA 77:3033–3037
Foote SL, Bloom FE, Aston-Jones G (1983) Nucleus locus coeruleus: new evidence of anatomical and physiological specifity. Physiol Rev 63:844–915
Furchgott RF (1972) The classification of adrenoceptors (adrenergic receptors). An evaluation from the standpoint of receptor theory. In: Blaschko H, Muscholl E (eds) Catecholamines. Handbook of Experimental Pharmacology Vol 33. Springer, Berlin Heidelberg, pp 283–335
Hagan RM, Hughes IE (1984) Opioid receptor sub-types involved in the control of transmitter release in cortex of the brain of the rat. Neuropharmacology 23:491–495
Henderson G, Pepper CM, Shefner SA (1982) Electrophysiological properties of neurones contained in the locus coeruleus and mesencephalic nucleus of the trigeminal nerve in vitro. Exp Brain Res 45:29–37
Illes P (1986) Mechanisms of receptor-mediated modulation of transmitter release in noradrenergic, cholinergic and sensory neurones. Neuroscience 17:909–928
Illes P (1989) Modulation of transmitter and hormone release by multiple neuronal opioid receptors. Rev Physiol Biochem Pharmacol 112:139–233
Illes P, Regenold JT (1990) Interaction between neuropeptide Y and noradrenaline on central catecholamine neurons. Nature 344:62–63
Illes P, Weber HD, Neuburger J, Bucher B, Regenold JT, Nörenberg W (1990) Receptor interactions at noradrenergic neurones. In: Kalsner S, Westfall TC (eds) Presynaptic receptors and the question of autoregulation of neurotransmitter release. Ann NY Acad Sci (in press)
Jackisch R, Geppert M, Illes P (1986) Characterization of opioid receptors modulating noradrenaline release in the hippocampus of the rabbit. J Neurochem 46:1802–1810
Langer SZ (1981) Presynaptic regulation of the release of catecholamines. Pharmacol Rev 32:337–361
Limberger N, Späth L, Hölting T, Starke K (1986) Mutual interaction between presynaptic α2-adrenoceptors and opioid к-receptors at the noradrenergic axons of rabbit brain cortex. Naunyn-Schmiedeberg's Arch Pharmacol 334:166–171
Limberger N, Singer EA, Starke K (1988a) Only activated but not non-activated presynaptic a2-autoreceptors interfere with neighbouring presynaptic receptor mechanisms. Naunyn-Schmiedeberg's Arch Pharmacol 338:62–67
Limberger N, Späth L, Starke K (1988b) Presynaptic α2-adrenoceptor, opioid x-receptor and adenosine A1-receptor interactions on noradrenaline release in rabbit brain cortex. Naunyn-Schmiedeberg's Arch Pharmacol 338:53–61
Lord JAH, Waterfield AA, Hughes J, Kosterlitz HW (1977) Endogenous opioid peptides: multiple agonists and receptors. Nature 267:495–499
Loughlin SE, Fallon JH (1985) Locus coeruleus. In: Paxinos G (ed) The rat nervous system, vol 2, Hindbrain and spinal cord. Academic Press, Sydney, pp 79–93
Marwaha J, Aghajanian GK (1982) Relative potencies of α1- and α2-antagonists in the locus ceruleus, dorsal raphe and dorsal lateral geniculate nuclei: an electrophysiological study. J Pharmacol Exp Ther 222:287–293
North RA (1986) Receptors on individual neurones. Neuroscience 17:899–907
North RA, Williams JT (1985) On the potassium conductance increased by opioids in rat locus coeruleus neurones. J Physiol 364:265–280
North RA, Williams JT, Surprenant A, Christie MJ (1987) μ and δ receptors belong to a family of receptors that are coupled to potassium channels. Proc Natl Acad Sci USA 84:5487–5491
Paterson SJ, Robson LE, Kosterlitz HW (1983) Classification of opioid receptors. Br Med Bull 39:31–36
Ramme D, Illes P, Späth L, Starke K (1986) Blockade of α2-adrenoceptors permits the operation of otherwise silent opioid κ-receptors at the sympathetic axons of rabbit jejunal arteries. Naunyn-Schmiedeberg's Arch Pharmacol 334:48–55
Regenold JT, Haas HL, Illes P (1988) Effects of purinoceptor agonists on electrophysiological properties of rat mesencephalic trigeminal neurones in vitro. Neurosci Lett 92:347–350
Schoffelmeer ANM, Putters J, Mulder AH (1986) Activation of presynaptic α2-adrenoceptors attenuates the inhibitory effect of μ-opioid receptor agonists on noradrenaline release from brain slices. Naunyn-Schmiedeberg's Arch Pharmacol 333:377–380
Snedecor GW, Cochran WG (1980) Statistical methods. Iowa State University Press, Ames
Starke K (1977) Regulation of noradrenaline release by presynaptic receptor systems. Rev Physiol Biochem Pharmacol 7:1–124
Surprenant A, Williams JT (1987) Inhibitory synaptic potentials recorded from mammalian neurones prolonged by blockade of noradrenaline uptake. J Physiol 382:87–103
Weitzell R, Tanaka T, Starke K (1979) Pre- and postsynaptic effects of yohimbine stereoisomers on noradrenaline transmission in the pulmonary artery of the rabbit. Naunyn-Schmiedeberg's Arch Pharmacol 308:127–136
Werling LL, McMahon PN, Cox BM (1989) Effects of pertussis toxin on opioid regulation of catecholamine release from rat and guinea pig brain slices. Naunyn-Schmiedeberg's Arch Pharmacol 339:509–513
Williams JT, North RA (1984) Opiate-receptor interactions on single locus coeruleus neurones. Mol Pharmacol 26:489–497
Williams JT, Egan TM, North RA (1982) Enkephalin opens potassium channels on mammalian central neurones. Nature 229:74–77
Williams JT, Henderson G, North RA (1985) Characterization of α2-adrenoceptors which increase potassium conductance in rat locus coeruleus neurones. Neuroscience 14:95–101
Williams JT, North RA, Tokimasa T (1988) Inward rectification of resting and opiate-activated potassium currents in rat locus coeruleus neurons. J Neurosci 8:4299–4306
Author information
Authors and Affiliations
Additional information
Send offprint requests to: P. Illes at the above address
Rights and permissions
About this article
Cite this article
Illes, P., Nörenberg, W. Blockade of α2-adrenoceptors increases opioid μ-receptor-mediated inhibition of the firing rate of rat locus coeruleus neurones. Naunyn-Schmiedeberg's Arch Pharmacol 342, 490–496 (1990). https://doi.org/10.1007/BF00169034
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00169034