Summary
Guinea-pig vasa deferentia or hypogastric nerve-vas deferens preparations, preincubated with pargyline (to irreversibly inhibit monoamine oxidase), were exposed to 2.3 μmol/l of unlabelled adrenaline or of 3H-7-(−)-noradrenaline in the presence of hydrocortisone (to inhibit extraneuronal uptake). The vasa deferentia were then mounted in perifusion chambers and subjected to transmural electrical stimulation, electrical stimulation of the nerve, depolarization by potassium (50 mmol/l), or addition of tyramine (40 μmol/l). The evoked overflow of tritium and of unlabelled catecholamines was expressed as a fraction of their tissue content. For all stimuli, the fractional release of the exogenous amines was higher than that of endogenous noradrenaline. Thus, recently incorporated amines are preferentially mobilized irrespective of the particular type of releasing mechanism or the chemical nature of the amine.
In vasa deferentia which had been loaded with increasing amounts of adrenaline (by incubating the tissues with adrenaline at concentrations ranging from 0.6 to 160 μmol/1), the fractional release of adrenaline decreased and became closer to that of endogenous noradrenaline. Hence, the access of exogenous catecholamines to the deepest storage sites requires higher concentrations of amines than those needed to reach the more easily releasable pools.
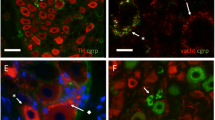
Light microscope autoradiographs obtained from slices of vasa deferentia previously loaded with 2.3 μmol/l 3H-(−)-noradrenaline showed that the outer layers were strongly labelled with silver grains whereas the inner layers were poorly marked. It is concluded that recently incorporated amines are preferentially stored in varicosities close to the surface of the tissue and, in comparison with endogenous noradrenaline, are preferentially released from sympathetically innervated organs.
Similar content being viewed by others
References
Brandão F, Rodrigues-Pereira E, Monteiro JG, Davidson R (1981) A kinetic study of the release of noradrenaline by tyramine. Naunyn-Schmiedeberg's Arch Pharmacol 318:83–87
Brandão F, Araújo D, Monteiro JG (1989) The influence of monoamine oxidase activity on the release of noradrenaline by tyramine. J Pharm Pharmacol 41:729–732
Crout JR (1964) The uptake and release of H3-norepinephrine by the guinea-pig heart in vivo. Naunyn-Schmiedeberg's Arch Pharmacol 248:85–98
Crout JR, Muskus AJ, Trendelenburg U (1962) Effect of tyramine on isolated guinea-pig atria in relation to their noradrenaline stores. Br J Pharmacol 18:600–611
Davies JC, Kissinger PT, Shoup RE (1981) Strategies for determination of serum of plasma norepinephrine by reverse-phase liquid chromatography. Anal Chem 53:156–159
De la Lande IS, Frewin D, Waterson JG (1967) The influence of sympathetic innervation on vascular sensitivity to noradrenaline. Br J Pharmacol 31:82–93
Graefe K-H, Stefano FJE, Langer SZ (1973) Preferential metabolism of (−)-3H-norepinephrine through the deaminated glycol in the rat vas deferens. Biochem Pharmacol 22:1147–1160
Guimarães S, Brandão F, Paiva MQ (1978) A study of the adrenoceptor-mediated feedback mechanisms by using adrenaline as a false transmitter. Naunyn-Schmiedeberg's Arch Pharmacol 305:185–188
Guimarães S, Moura D, Paiva MQ (1986) Effects of phentolamine on the release of [3H]-(−)- and endogenous noradrenaline by direct electrical stimulation. Br J Pharmacol 89:838P
Hughes J (1973) Differential labelling of intraneuronal noradrenaline stores with different concentrations of (−)-3H-noradrenaline. Br J Pharmacol 47:428–430
Hukovic S (1961) Responses of the isolated sympathetic nerve-ductus deferens preparation of the guinea pig. Br J Pharmacol Chemother 16:188–194
Iversen LL (1965) The uptake of adrenaline by the rat isolated heart. Br J Pharmacol 24:387–394
Langeloh A, Trendelenburg U (1987) The mechanism of the 3H-noradrenaline releasing effect of various substrates of uptake1: role of monoamine oxidase and of vesicularly stored 3H-noradrenaline. Naunyn-Schmiedeberg's Arch Pharmacol 336:611–620
Langer SZ, Lehmann J (1988) Presynaptic receptors on catecholamine neurones. In: Trendelenburg U, Weiner N (eds) Catecholamines I, Handbook of Experimental Pharmacology, Vol 90/I. Springer, Berlin Heidelberg New York Tokyo, pp 419–507
Luchelli-Fortis MA, Langer SZ (1975) Selective inhibition by hydrocortisone of 3H-normetanephrine formation during 3H-transmitter release elicited by nerve stimulation in the isolated nerve-muscle preparation of the cat nictitating membrane. Naunyn-Schmiedeberg's Arch Pharmacol 287:261–275
Majewski H, McCullogh MW, Rand MJ, Story DF (1980) Adrenaline activation of pre junctional \-adrenoceptors in guinea-pig atria. Br J Pharmacol 71:435–444
Moura D, Paiva MQ, Guimarães S (1984a) Release of noradrenaline from dog saphenous vein strips previously incubated with adrenaline. Blood Vessels 21:141–142
Moura D, Paiva MQ, Guimarães S (1984b) Comparison of 3H-noradrenaline (3H-NA) and endogenous noradrenaline (NA) release from guinea-pig vas deferens by electrical stimulation. IUPHAR 9th Int Congr Pharmacol, London (Abstracts) 372P
Schömig E, Halbrügge T, Schönfeld C-L, Graefe K-H, Trendelenburg U (1990) The inhomogeneity of the distribution of neuronal 3H-noradrenaline (3H-NA) in the rat vas deferens. Naunyn-Schmiedeberg's Arch Pharmacol 342:160–170
Starke K, Göthert M, Kilbinger H (1989) Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol Rev 69:864–989
Stjärne L (1975) Selectivity for catecholamines of presynaptic alpha-receptors involved in feedback control of sympathetic neurotransmitter secretion in guinea-pig vas deferens. Naunyn-Schmiedeberg's Arch Pharmacol 288:296–303
Trendelenburg U (1961) Modification of the effect of tyramine by various agents and procedures. J Pharmacol Exp Ther 134:8–17
Trendelenburg U (1972) Factors influencing the concentration of catecholamines at the receptors. In: Blaschko H, Muscholl E (eds) Catecholamines. Handbook of Experimental Pharmacology, Vol 33. Springer, Berlin Heidelberg New York, pp 726–761
Trendelenburg U, Stefano FJE, Grohmann M (1983) The isotope effect of tritium in 3H-noradrenaline. Naunyn-Schmiedeberg's Arch Pharmacol 323:128–140
Wakade AR, Kirpekar SM (1971) Chemical and histochemical studies on the sympathetic innervation of the vas deferens and seminal vesicle of the guinea pig. J Pharmacol Exp Ther 178:432–441
Warnhoff M (1984) Simultaneous determination of norepinephrine, dopamine, 5-hydroxytryptamine and their main metabolites in rat brain using high-performance liquid chromatography with electrochemical detection. J Chromatogr 307:271–281
Author information
Authors and Affiliations
Additional information
Send offprint requests to D. Moura at the above address
Rights and permissions
About this article
Cite this article
Moura, D., Azevedo, I. & Guimarães, S. Differential distribution in, and release from, sympathetic nerve endings of endogenous noradrenaline and recently incorporated catecholamines. Naunyn-Schmiedeberg's Arch Pharmacol 342, 153–159 (1990). https://doi.org/10.1007/BF00166958
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00166958