Abstract
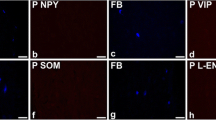
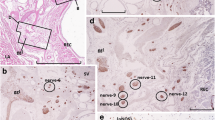
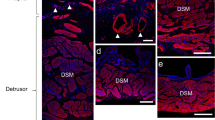
Bladder afferent outflow, linked to sensation, plays a critical role in bladder pathology: abnormal outflow results in altered sensation, leading to increased voiding frequency, urge and often incontinence. β3-adrenoceptor agonists have been suggested to be beneficial in treating these symptoms. However, the absence of a significant sympathetic innervation of the detrusor and only a modest relaxation of bladder muscle by β3 agonists has questioned the therapeutic site of action of β3 agonists in the bladder. The present study was done to explore the possibility that β3-adrenoceptors might be located in the pelvic plexus. Using the rat, where the pelvic plexus is located primarily within a single ganglion, the major pelvic ganglion (MPG), immuno-histochemical approaches were used to identify structures expressing β3-adrenoceptor immuno-reactivity (β3AR-IR). The only structures found to express β3AR-IR were small-diameter tyrosine hydroxylase and vesicular mono-amine transporter immuno-reactive (TH-IR and vmat-IR) neurones. These neurones, found in clusters or singly on the periphery of the ganglion, or dispersed in smaller clumps throughout the MPG, are similar to the small intensely fluorescent (SIF) cells described previously. Not all small cells expressed β3AR-IR. A population of the small cells were also immuno-reactive to the type 3 muscarinic receptor (M3R-IR) and the P2X3 purinergic receptor (P2X3-IR). Clumps of small cells were associated with calcitonin gene-related peptide immuno-reactive (CGRP-IR) nerve fibres (putative sensory fibres) and a small number were contacted by putative cholinergic nerves expressing immuno-reactivity to vesicular acetylcholine transporter (vacht-IR). These observations are consistent with the idea that small cells are interneurons and one of the components making up complex neural circuits within the MPG. The precise physiological role of these neural elements in the MPG is unknown. However, as one therapeutic action of β3-adrenoceptor agonists is to modulate sensation, it is possible that these neural circuits may be involved in the regulation of afferent outflow and sensation.










Similar content being viewed by others
References
Cernecka H, Pradidarcheep W, Lamers WH, Schmidt M, Michel MC (2014) Rat beta(3)-adrenoceptor protein expression: antibody validation and distribution in rat gastrointestinal and urogenital tissues. Naunyn Schmiedebergs Arch Pharmacol 387(11):1117–1127
Chapple C (2014) Mirabegron the first beta3 -adrenoceptor agonist for overactive bladder (OAB): a summary of the phase III studies. BJU Int 113(6):847–848
Chapple CR, Nitti VW, Khullar V, Wyndaele JJ, Herschorn S, van Kerrebroeck P et al. (2014) Onset of action of the beta3-adrenoceptor agonist, mirabegron, in Phase II and III clinical trials in patients with overactive bladder. World J Urol 32:1565–1572
Crowcroft PJ, Holman ME, Szurszewski JH (1971) Excitatory input from the distal colon to the inferior mesenteric ganglion in the guinea-pig. J Physiol 219(2):443–461
Dail WG Jr, Evan AP Jr, Eason HR (1975) The major ganglion in the pelvic plexus of the male rat—a histochemical and ultrastructural study. Cell Tissue Res 159(1):49–62
De Groat W, Booth AM (1993) Synaptic transmission in pelvic ganglia. In: Nervous control of the urogenital system. CA Maggi editor: Harwood academic publishers
de Groat WC, Booth AM (1980) Inhibition and facilitation in parasympathetic ganglia of the urinary bladder. Fed Proc 39(12):2990–2996
Dixon JS, Gilpin SA, Gilpin CJ, Gosling JA (1983) Intramural ganglia of the human urinary bladder. Br J Urol 55(2):195–198
Dixon JS, Gosling JA, Canning DA, Gearhart JP (1992) An immunohistochemical study of human postnatal paraganglia associated with the urinary-bladder. J Anat 181:431–436
Eastham JE, Gillespie JI (2013) The concept of peripheral modulation of bladder sensation. Organogenesis 9(3):177–186
el-Badawi A, Shenk EA (1968) A new theory of the innervation of bladder musculature. 1. Morphology of the intrinsic vesical innervation apparatus. J Urol 99(5):585–587
El-Badawi A, Schenk EA (1968) The peripheral adrenergic innervation apparatus - I. Intraganglionic and extraganglionic adrenergic ganglion cells. Zeitschrift für Zellforschung und Mikroskopische Anatomie 87(2):218–225
Eränkö O (1976) SIF cells, chromaffin cells, granule-containing cells, and interneurons. In: Eränkö O (ed) SIF cells structure and function of the small, intensely fluorescent sympathetic cells. Raven Press, New York, pp 1–7
Eränkö O (1978) Small intensely fluorescent (SIF) cells and nervous transmission in sympathetic ganglia. Annu Rev Pharmacol Toxicol 18:417–430
Fujimura T, Tamura K, Tsutsumi T, Yamamoto T, Nakamura K, Koibuchi Y et al (1999) Expression and possible functional role of the β3-adrenoceptor in human and rat detrusor muscle. J Urol 161(2):680–685
Gabella G (1990) Intramural neurons in the urinary-bladder of the guinea-pig. Cell Tissue Res 261(2):231–237
Gillespie JI (2004) The autonomous bladder: a view of the origin of bladder overactivity and sensory urge. BJU Int 93(4):478–483
Gillespie JI, Markerink-Van Ittersum M, De Vente J (2006) Sensory collaterals, intramural ganglia and motor nerves in the guinea-pig bladder: evidence for intramural neural circuits. Cell Tissue Res 325(1):33–45
Gillespie JI, Rouget C, Palea S, C. G, Korstanje C (2015) Beta adrenergic modulation of spontaneous microcontractions and electrical field-stimulated contractions in isolated strips of the rat bladder from normal animals and animals with partial bladder outflow obstruction NSAP - Submitted Ref No -NSAP-D-14-00220
Gillespie JI, Palea S, Guilloteau V, Guerard M, Lluel P, Korstanje C (2012) Modulation of non-voiding activity by the muscarinergic antagonist tolterodine and the beta(3)-adrenoceptor agonist mirabegron in conscious rats with partial outflow obstruction. BJU Int 110(2 Pt 2):E132–E142
Gillespie JI, Rouget C, Palea S, Granato C, Birder L, Korstanje C (2015). The characteristics of intrinsic complex micro-contractile activity in isolated strips of the rat bladder NSAP - Submitted Ref No -NSAP-D-14-00219
Gosling JA, Dixon JS (1974) Sensory nerves in mammalian urinary-tract—evaluation using light and electron-microscopy. J Anat 117:133–144
Gosling JA, Dixon JS, Jen PYP (1999) The distribution of noradrenergic nerves in the human lower urinary tract. Rev Eur Urol 36(1):23–30
Grol S, Essers PBM, Van Koeveringe GA, Martinez-Martinez P, De Vente J, Gillespie JI (2009) M 3 muscarinic receptor expression on suburothelial interstitial cells. BJU Int 104(3):398–405
Igawa Y, Yamazaki Y, Takeda H, Akahane M, Ajisawa Y, Yoneyama T et al (1998) Possible β3-adrenoceptor-mediated relaxation of the human detrusor. Acta Physiol Scand 164(1):117–118
Iggo A (1955) Tension receptors in the stomach and the urinary bladder. J Physiol 128(3):593–607
Keast JR (1995) Visualization and immunohistochemical characterization of sympathetic and parasympathetic neurons in the male rat major pelvic ganglion. Neuroscience 66(3):655–662
Keast JR, Booth AM, De Groat WC (1989) Distribution of neurons in the major pelvic ganglion of the rat which supply the bladder, colon or penis. Cell Tissue Res 256(1):105–112
Krauwinkel W, van Dijk J, Schaddelee M, Eltink C, Meijer J, Strabach G et al (2012) Pharmacokinetic properties of mirabegron, a β3-adrenoceptor agonist: results from two phase I, randomized, multiple-dose studies in healthy young and elderly men and women. Clin Ther 34(10):2144–2160
Langley JN, Anderson HK (1895a) The innervation of the pelvic and adjoining viscera. Part 1. The lower portion of the intestine. J Physiol(London) 18(:67–105
Langley JN, Anderson HK (1895b) The innervation of the pelvic and adjoining viscera. Part 2. The bladder. J Physiol(London) 19:71–84
Langley JN, Anderson HK (1896) The innervation of the pelvic and adjoining viscera. Part 7. Anatomical observations. J Physiol(London) 20:372–406
Langworthy OR (1965) Innervation of the pelvic organs of the rat. Invest Urol 2:491–511
Owman C, Alm P, Sjoberg N (1983) Pelvic autonomic ganglia: structure, transmitters, function and steroid influence. In: Elfvin LG (ed) Autonomic Ganglia. John Wiley and Sons Ltd, New York
Sacco E (2014) Discovery history and clinical development of mirabegron for the treatment of overactive bladder and urinary incontinence. Expert Opin Drug Discov EPub Ahead of print
Sacco E, Bientinesi R (2012) Mirabegron: a review of recent data and its prospects in the management of overactive bladder. Ther Adv Urol 4(6):315–324
Sadananda P, Drake M, Paton J, Pickering A (2013) A functional analysis of the influence of beta 3-adrenoceptors on the rat micturition cycle. J Pharmacol Exp Ther 347:506–515
Seguchi H, Nishimura J, Zhou Y, Niiro N, Kumazawa J, Kanaide H (1998) Expression of β3-adrenoceptors in rat detrusor smooth muscle. J Urol 159(6):2197–2201
Sherrington CS (1892) Notes on the arrangement of motor fibers in the lumbo-sacral plexus. J Physiol 13:621–772
Smet PJ, Edyvane KA, Jonavicius J, Marshall VR (1996) Neuropeptides and neurotransmitter-synthesizing enzymes in intrinsic neurons of the human urinary bladder. J Neurocytol 25(2):112–124
Starling EH (1905) Elements of human physiology, 7th edn. London, Churchill
Sugaya K, de Groat WC (2007) Bladder volume-dependent excitatory and inhibitory influence of lumbosacral dorsal and ventral roots on bladder activity in rats. Biomed Res 28(4):169–175
Sugaya K, De Groat WC (2009) Excitatory and inhibitory influence of pathways in the pelvic nerve on bladder activity in rats with bladder outlet obstruction. Lower Urinary Tract Symptoms 1(1):51–55
Svalø J, Nordling J, Bouchelouche K, Andersson KE, Korstanje C, Bouchelouche P (2013) The novel β3-adrenoceptor agonist mirabegron reduces carbachol-induced contractile activity in detrusor tissue from patients with bladder outflow obstruction with or without detrusor overactivity. Eur J Pharmacol 699(1-3):101–105
Szurszewski JH (1981) Physiology of mammalian prevertebral ganglia. Ann Rev Physiol 43:53–68
Szurszewski JH, Ermilov LG, Miller SM (2002) Prevertebral ganglia and intestinofugal afferent neurones. Gut 51(1):i6–i10
Takeda H, Yamazaki Y, Igawa Y, Kaidoh K, Akahane S, Miyata H et al (2002) Effects of β3-adrenoceptor stimulation on prostaglandin E2-induced bladder hyperactivity and on the cardiovascular system in conscious rats. Neurourol Urodyn 21(6):558–565
Taxi J, Derer M, Domich A (1983) Morphology and histophysiology of SIF cells in the autonomic ganglia. In: Elfin L (ed) Autonomic ganglia. John Wiley and Sons Ltd, New York, pp 67–95
Uvelius B, Gabella G (1995) Intramural neurones appear in the urinary bladder wall following excision of the pelvic ganglion in the rat. NeuroReport 6(16):2213–2216
Vaughan CW, Satchell PM (1995) Urine storage mechanisms. Prog Neurobiol 46(2-3):215–237
Watanabe H, Yamamoto TY (1979) Autonomic innervation of the muscles in the wall of the bladder and proximal urethra of male rats. J Anat 128(4):873–886
Wilson PO, Barber PC, Hamid QA, Power BF, Dhillon AP, Rode J et al (1988) The immunolocalization of protein gene product 9.5 using rabbit polyclonal and mouse monoclonal antibodies. Br J Exp Pathol 69(1):91–104
Zhou Y, Ling EA (1998) Colocalization of nitric oxide synthase and some neurotransmitters in the intramural ganglia of the guinea pig urinary bladder. J Comp Neurol 394(4):496–505
Compliance with ethical standards
This manuscript has been prepared to comply with all required ethical standards. There are no conflicts of interest to be declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eastham, J., Stephenson, C., Korstanje, K. et al. The expression of β3-adrenoceptor and muscarinic type 3 receptor immuno-reactivity in the major pelvic ganglion of the rat. Naunyn-Schmiedeberg's Arch Pharmacol 388, 695–708 (2015). https://doi.org/10.1007/s00210-015-1122-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-015-1122-5