Abstract
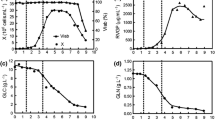
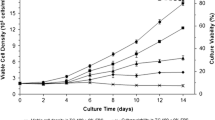
Insect cell metabolism was studied in substrate-limited fed batch cultures of Spodoptera frugiperda (Sf-9) cells. Results from a glucose-limited culture, a glutamine-limited culture, a culture limited in both glucose and glutamine and a batch culture were compared. A stringent relation between glucose excess and alanine formation was found. In contrast, glucose limitation induced ammonium formation, while, at the same time, alanine formation was completely suppressed. Simultaneous glucose and glutamine limitation suppressed both alanine and ammonium formation. Although the metabolism was influenced by substrate limitation, the specific growth rate was similar in all cultures. Alanine formation must involve incorporation of free ammonium, if ammonium formation is mediated by glutaminase and glutamate dehydrogenase, as our data suggest. On the basis of the results, two possible pathways for the formation of alanine in the intermediary metabolism in insect cells are suggested. The cellular yield on glucose was increased 6.6 times during glucose limitation, independently of the cellular yield on glutamine, which was increased 50–100 times during glutamine limitation. The results indicate that alanine overflow metabolism is energetically wasteful and that glutamine is a dispensable amino acid for cultured Sf-9 cells. Preliminary data confirm that glutamine can be synthesised by the cells themselves in amounts sufficient to support growth.
Similar content being viewed by others
References
Atkinson DE (1977) Cellular energy metabolism and its regulation. Academic Press, New York
Barkhem T, Carlsson B, Danielsson A, Norinder U, Frieberg H, Öhman L (1992) Production in a 100 liter stirred tank reactor of functional full length human thyroid receptor β1 in Sf-9 insect cells using a recombinant baculovirus. In: Vlak JM, Schlaeger E-J, Bernard AR (eds) Baculovirus and recombinant protein production processes. Proceedings of the baculovirus and recombinant protein production workshop, March 29–April 1, Interlaken, Editiones Roche, Basel, Switzerland, pp 235–246
Bédard C, Tom R, Kamen A (1993) Growth, nutrient consumption, and end-product accumulation in Sf-9 and BTI-EAA insect cell cultures: insights into growth limitation and metabolism. Biotechnol Prog 9:615–624
Caron AW, Archambault J, Massie B (1990) High-level recombinant protein production in bioreactors using the baculovirus-insect cell expression system. Biotechnol Bioeng 36:1133–1140
Ferrance JP, Goel A, Ataai MM (1993) Utilization of glucose and amino acids in insect cell cultures: Quantifying the metabolic flows within the primary pathways and medium development. Biotechnol Bioeng 42:697–707
Hashimoto S, Katsumata R (1993) Overproduction of alanine by Arthrobacter strains with glucose-nonrepressible l-alanine dehydrogenase. Biotechnol Lett 15:1117–1122
Kitai A (1972) Alanine. In: Yamada K, Kinoshita, Tsunoda T, Aida K (eds) The microbial production of amino acids. Halsted/Kodanasha, Tokyo, pp 325–337
Kovacevic Z, McGivan JD (1983) Mitochondrial metabolism of glutamine and glutamate and its physiological significance. Physiol Rev 63:547–605
Lin A, Agrawal P (1988) Glutamine decomposition in DMEM: effect of pH and serum concentration. Biotechnol Lett 10:695–698
Lindsay DA, Betenbaugh MJ (1992) Quantification of cell culture factors affecting recombinant protein yields in baculovirus-infected cells. Biotechnol Bioeng 39:614–618
Ljunggren J, Häggström L (1994) Catabolic control of hybridoma cells by glucose and glutamine limited fed batch cultures. Biotechnol Bioeng 44:808–818
Martinelle K, Häggström L (1993) Mechanisms of ammonia and ammonium ion toxicity in animal cells: transport across cell membranes. J Biotechnol 30:339–350
McCowen SM, Phibbs J PV (1974) Regulation of alanine dehydrogenase in Bacillus licheniformis. J Bacteriol 118:590–597
Meister A (1955) Transamination. Adv Enzymol 16:185–246
Mitsuhashi J (1980) Requirements of amino acids by insect cell lines. In: Kurtstak E, Marmorosch K, Dübendorfer A (eds) Invertebrate systems in vitro. Elsevier, North-Holland, pp 47–58
Ohashima T, Soda K (1979) Purification and properties of alanine dehydrogenase from Bacillus sphaericus. Eur J Biochem 100:29–39
Radford K, Reid S, Greenfield P (1992) Improved production of recombinant proteins by the baculovirus expression system using nutrient enriched serum free media. In: Vlak JM, Schlaeger E-J, Bernard AR (eds) Baculovirus and recombinant protein production processes. Proceedings of the baculovirus and recombinant protein production workshop, March 29–April 1, Interlaken, Switzerland. pp 297–303
Rekharsky MV, Galchenko GL, Egorv AM, Berenzin IV (1986) Thermodynamics of enzymatic reactions. In: Hinz H-J (ed) Thermodynamic data for biochemistry and biotechnology. Springer, Berlin Heidelberg, pp 431–444
Reuveny S, Kemp CW, Eppstein L, Shiloach J (1992) Carbohydrate metabolism in insect cell cultures during cell growth and recombinant protein production. Ann N Y Acad Sci 665:230–237
Reuveny S, Kim YJ, Kemp CW, Shiloach J (1993) Production of recombinant proteins in high-density insect cell cultures. Biotechnol Bioeng 42:235–239
Wang M-Y, Vakharia V, Bentley WE (1993) Expression of epoxide hydrolase in insect cells: a focus on the infected cell. Biotechnol Bioeng 42:240–246
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Öhman, L., Ljunggren, J. & Häggström, L. Induction of a metabolic switch in insect cells by substrate-limited fed batch cultures. Appl Microbiol Biotechnol 43, 1006–1013 (1995). https://doi.org/10.1007/BF00166917
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00166917