Summary
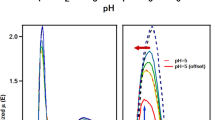
CoII chloro complexes were studied in MeOH at 25 °C and at constant ionic strength of 1 mol dm−3. Formation of three complexes is postulated for which the overall stability constants are calculated: logβ 1 = 1.2, log β 2 = 1.7 and log β 3 = 1.4. The electronic spectra and the formation curves of the identified species are presented for the first time in this medium. The results are compared with those obtained in other alcohols and increasing stability with increasing molecular weight of the solvents is established. Further comparative study showed that the maximum stability of the chloro complexes is found with the CuII ion as the central atom. This confirms the Irving-Williams order of stabilities for the first transition metal complexes in this alcoholic medium and the result is explained in terms of the second ionization potential of the elements.
Similar content being viewed by others
References
S. Lechat, M. A. Khan, G. Bouet and F. Vierling, Inorg. Chim. Acta, 211, 33 (1993).
L. I. Katzin and E. Gebert, J. Amer. Chem. Soc., 72, 5464 (1950).
D. A. Fine, J. Amer. Chem. Soc., 84, 1140 (1962).
F. A. Cotton, D. M. L. Goodgame and M. Goodgame, J. Amer. Chem. Soc., 83, 4690 (1961).
E. Wendling, O. Benali-Baïtich and G. Yaker, Rev. Chim. Miner., 8, 559 (1971).
A. H. Zeltman, N. A. Tiatwiyoff and L. O. Morgan, J. Phys. Chem., 72, 121 (1968).
J. Bjerrum, A. S. Halonin and L. H. Skibsted, Acta Chem. Scand., Ser. A, 32, 429 (1978).
A. Chiboub Fellah, Doctoral Thesis, Université Louis Pasteur, Strasbourg, January (1991).
Z. Z. Hugus and A. A. El Awady, J. Phys. Chem., 75(19), 2954 (1971).
F. Vierling, M. J. Schwing and J. Meullemeestre, Spectra 2000, 79(10), 25 (1982).
J. Byé, R. Hugel, G. Schorsch and R. Strosser, Bull. Soc. Chim. Fr., 1146 (1964).
D. W. Marquardt, J. Soc. Ind. Appl. Math., 11(2), 431 (1963).
E. Bentouhami, M. A. Khan, J. Meullemeestre and F. Vierling, Polyhedron., 11(17), 2179 (1992).
M. A. Khan, D. Cronier, G. Bouet and F. Vierling, Transition Met. Chem., 20, 369 (1995).
M. A. Khan, J. Meullemeestre, M. J. Schwing and F. Vierling, Inorg. Chem., 28, 3306 (1989).
S. Chafaa, T. Douadi, M. A. Khan, J. Meullemeestre, M. J. Schwing and F. Vierling, Nouv. J. Chim., 15, 39 (1991).
F. Djabi, J. Meullemeestre, F. Vierling, G. Bouet and M. A. Khan, Bull. Soc. Chim. Fr., 131, 53 (1994).
H. Irving and R. J. P. Williams, J. Chem. Soc., 3192 (1953).
R. B. Heslop and P. L. Robinson, Chimie Inorganique, Flammarion Sciences, Paris, 1973, p. 597.
H. J. Emeleus and J. S. Anderson, Modern Aspects of Inorganic Chemistry, 3rd Edition, ELBS, London, 1962, p. 86.
F. A. Cotton and G. Wilkinson, Advanced Inorganic Chemistry, 5th Edit., Wiley, New York, 1988, Appendix 4, ionic radii, p. 1388.
A. F. Wells, Structural Inorganic Chemistry, 5th Edit. Clarendon Press, Oxford, 1987, p. 313.
Handbook of Chemistry and Physics, 69th Edit. CRC Press, Boca Raton, 1988–89, pp. E-78, and F-164.
P. W. Atkins, Physical Chemistry, 4th Edit., Oxford University Press, Oxford, 1990, p. 959.
H. B. Gray and G. P. Haight, Principes de Chimie, Ediscience, Paris, 1973, p. 69.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Khan, M., Bouet, G., Vierling, F. et al. Formation of cobalt(II), nickel(II) and copper(II) chloro complexes in alcohols and the Irving-Williams order of stabilities. Transition Met Chem 21, 231–234 (1996). https://doi.org/10.1007/BF00165973
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00165973