Summary
-
1.
The effect of adenosine or its stable analogues (2-chloroadenosine, CADO; 5\t'-N-ethylcarboxamidoadenosine, NECA; and N6-cyclopentyladenosine, CPA) on the release of [3H]-acetylcholine ([3H]-ACh), and on the development of force of contraction evoked by electrical stimulation of the nerve, were studied in the mouse phrenic nerve-hemidiaphragm preparation. Evidence was obtained that the release of ACh is subject to presynaptic modulation through presynaptic A1(P1)-purinoceptors.
-
2.
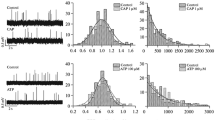
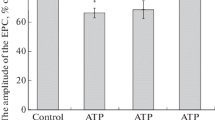
Adenosine or its stable analogues (CADO, NECA, CPA) inhibited, in a concentration-dependent manner, both the release of ACh and the force of the indirectly elicited contraction of hemidiaphragm preparation, provided in the latter case that the margin of safety was reduced by (\s+)-tubocurarine or magnesium. The order of potency in reducing ACh release was CPA > NECA > CADO > adenosine with IC50 values of 0.08 \+- 0.01, 0.74 \+- 0.05, 9.05 \+- 0.20, and 410.2 \+- 42.5 \gmmol/l, respectively. The order of potency in reducing twitch tension was CPA > NECA > CADO > adenosine with IC50 values of 0.11 \+- 0.02, 0.48 \+- 0.03, 2.07 \+- 0.49, and 240.4 \+- 20.0 \gmol/l, respectively.
-
3.
8-Phenyltheophylline (8-PT) antagonized the inhibitory effects of the adenosine receptor agonists on ACh release and twitch tension. The finding that -log KB and pA2 values estimated for 8-PT on ACh release and twitch tension were similar when adenosine or its stable analogues were applied as agonists suggest (i) that the motor nerve terminals are equipped with inhibitory P1-purinoceptors and (ii) that twitch responses of the partially curarized phrenic nerve-hemidiaphragm preparation to electrical stimulation can be used for studying the presynaptic inhibitory effect of P1-purinoceptor agonists. The findings that 8-PT and 8-cyclopentyl-1,3dipropylxanthine (DPCPX), a selective A1-purinoceptor antagonist, enhanced the release of [3H]-ACh in response to nerve stimulation and DPCPX antagonized the inhibitory effect of CADO on twitch tension (pA2 = 10.77 \+- 1.34) indicate that these receptors are of A1-subtype.
-
4.
Dipyridamole, an adenosine uptake blocker, reduced the release of [3H]-ACh, and reinforced the effect of adenosine, but did not affect responses to adenosine analogues not taken up by the tissue.
-
5.
It is concluded that the cholinergic axon terminals located at the neuromuscular junction are equipped with inhibitory A1-purinoceptors. Endogenous adenosine derived either from ATP or directly from the muscle or nerve terminals might thus activate these receptors to inhibit ACh release and thereby presynaptically influences chemical neurotransmission at the neuromuscular junction.
Similar content being viewed by others
References
Arunlakshana O, Schild HO (1959) Some quantitative uses of drug antagonists. Br J Pharmacol Chemother 14:48–58
Bowman WC (1990) Pharmacology of neuromuscular function. John Wiley, Bristol
Chen H, Singh YN, Dryden WF (1989) Transduction mechanism involving the presynaptic adenosine receptor at mouse motor nerve terminals. Neurosci Lett 96:318–322
Foldes FF (1981) The significance of physiological Ca2+ and Mg2+ for in vitro experiments on synaptic transmission. Life Sci 28:1585–1590
Ginsborg BL, Hirst GDS (1972) The effect of adenosine on the release of the transmitter from the phrenic nerve of the rat. J Physiol Lond 224:629–645
Gordon JL (1986) Extracellular ATP: effects, sources and fate. Biochem J 233:309–319
Gustafsson LE, Hedgvist P, Fredholm BB, Lundgren G (1978) Inhibition of acetylcholine release in guinea pig ileum by adenosine. Acta Physiol Scand 104:469–478
Haleen SJ, Steffen RP, Hamilton HW (1987) PD 116,948, a highly selective A1 adenosine receptor antagonist. Life Sci 40:555–561
Hamilton BR, Smith DO (1991) Autoreceptor-mediated purinergic and cholinergic inhibition of motor nerve terminal calcium currents in the rat. J Physiol Lend 432:327–341
Kolassa N, Pfleger K, Rummel W (1970) Specificity of adenosine uptake into the heart and inhibition by dipyridamole. Eur J Pharmacol 9: 265–268
Lohse MJ, Klotz KN, Lindenborn-Fotinos J, Reddington M, Schwabe U, Olson RA (1987) 8-Cyclopentyl-1,3-dipronyl-xanthine (DPCPX), selective high affinity antagonist radioligand for A1 adenosine receptors. Naunyn-Schmiedeberg's Arch Pharmacol 336:204–210
Nagy JI, Geiger JD, Staines WA (1990) Adenosine deaminase and purinergic neuroregulation. Neurochem Int 16:211–221
Paton WDM, Waud DR (1967) The margin of safety of neuromuscular transmission. J Physiol 191: 59–90
Ribeiro JA, Sebastiao AM (1985) On the type of receptor involved in the inhibitory action of adenosine at the neuromuscular junction. Br J Pharmacol 84:911–918
Ribeiro JA, Walker J (1975) The effects of adenosine triphosphate and adenosine diphosphate on transmission at the rat and frog neuromuscular junction. Br J Pharmacol 54:213–218
Sebastiao AM, Ribeiro JA (1988) On the adenosine receptor and adenosine inactivation at the rat diaphragm neuromuscular junction. Br J Pharmacol 94:109–120
Silinsky EM (1973) Release of ATP from rat motor nerve terminals. Nature 243:404–405
Silinsky EM (1975) On the association between transmitter secretion and the release of adenine nucleotides from mammalian motor nerve terminals. J Physiol Lond 247:145–162
Silinsky EM (1984) On the mechanism by which adenosine receptor activation inhibits the release of acetylcholine from motor nerve endings. J Physiol Lond 346:243–256
Singh YN, Dryden WF, Chen H (1986) The inhibitory effects of some adenosine analogues on transmitter release at the mammalian neuromuscular junction. Can J Physiol Pharmacol 64:1446–1450
Smith DO (1991) Sources of adenosine released during neuromuscular transmission in the rat. J Physiol Lond 432:343–354
Somogyi GT, Vizi ES (1988) Evidence that cholinergic axon terminals are equipped with both muscarinic and adenosine receptors. Brain Res Bull 21:575–579
Somogyi GT, Vizi ES, Chaudhry IA, Nagashima H, Duncalf D, Foldes FF, Goldiner PL (1987) Modulation of stimulation-evoked release of newly formed acetylcholine from mouse hemidiaphragm preparation. Naunyn-Schmiedeberg's Arch Pharmacol 336:11–15
Vizi ES (1989) In favour of the vesicular hypothesis: neurochemical evidence that vesamicol (AH 5183) inhibits stimulation-evoked release of acetylcholine from neuromuscular junction. Br J Pharmacol 98: 898–902
Vizi ES (1991) Evidence that catecholamines increase acetylcholine release from neuromuscular junction through stimulation of alpha-1 adrenoceptors. Naunyn-Schmiedeberg's Arch Pharmacol 343:435–438
Vizi ES, Knoll J (1976) The inhibitory effect of adenosine and related nucleotides on the release of acetylcholine. Neuroscience 1:391–398
Wessler I, Kilbinger H (1986) Release of (3H)acetylcholine from a modified rat phrenic nerve-hemidiaphragm preparation. Naunyn-Schmiedeberg's Arch Pharmacol 334:357–364
Wessler I, Steinlein O (1987) Differential release of 3H-acetylcholine from the rat phrenic nerve-hemidiaphragm preparation by electrical nerve stimulation and by high potassium. Neuroscience 22:289–299
Author information
Authors and Affiliations
Additional information
Send offprint requests to E. S. Vizi at the above address
Rights and permissions
About this article
Cite this article
Nagano, O., Földes, F.F., Nakatsuka, H. et al. Presynaptic A1-purinoceptor-mediated inhibitory effects of adenosine and its stable analogues on the mouse hemidiaphragm preparation. Naunyn-Schmiedeberg's Arch Pharmacol 346, 197–202 (1992). https://doi.org/10.1007/BF00165301
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00165301