Summary
Effects of adenosine and nucleotides on the release of previously stored [3H]-noradrenaline were studied in rabbit brain cortex slices. The slices were stimulated twice, in most experiments by 6 electrical field pulses delivered at 100 Hz.
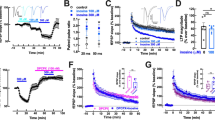
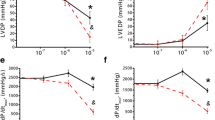
Adenosine and the nucleotides AMP, ADP, ATP, AMPS, ADPβS, ATPyS, β,γ-imido-ATP and β,γ-methyl-ene-ATP all reduced the evoked overflow of tritiated compounds. For purines for which concentration-response curves were determined, the order of potency was adenosine > ATP ≈ ATPyS β,γ-imido-ATP ≈ ADP > β,γ-methylene-ATP. AMP 30 Etmol/l and AMPS 30 μmol/l were approximately equieffective with 30 μmol/l of adenosine and ATPγS, and ADPβS, 30 μmol/l was approximately equieffective with 30 μmol/l of ADP. α,β-Methylene-ADP, 2-methylthio-ATP, UTP and GTPγS did not change the evoked overflow of tritium. α,β-Methylene-ATP caused an increase; however, the increase was small and became significant only after 59 min of exposure to α,β-methylene-ATP or when the slices were stimulated by 30 pulses, 10 H2. Neither adenosine deaminase (100 U/l) nor the blocker of 5′-nucleotidase, α,β-methylene-ADP (10 μmol/l), attenuated the inhibition caused by ATP, ATPyS and β,γ-methylene-ATP, despite the fact that adenosine deaminase abolished the effect of adenosine. 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX, 10 nmol/l) shifted the concentration-response curves of adenosine, ATPyS, β,γ-imido-ATP and β,γ-methylene-ATP to the right by very similar degrees. 8(p-Sulphophenyl)-theophylline (30 and 300 μmol/l) also markedly antagonized the inhibition produced by ATPγS. α,β-Methylene-ATP (10 and 30 μmol/l) and suramin (100 gmol/l) did not modify the effects of adenosine, ATPγS and β,γ-methylene-ATP.
It is concluded that nucleotides themselves can inhibit the release of noradrenaline in the rabbit brain cortex. The nucleotides and adenosine seem to act at the same site, i.e., the A1 subtype of the P1-purinoceptor. The results support the notion that metabolically stable, phosphate chain-modified nucleotides such as ATPγS, β,γ-imido-ATP and β,γ-methylene-ATP can be potent P1 agonists. No evidence was found for presynaptic P2X-, P2Y- or P3-purinoceptors.
Similar content being viewed by others
References
Allgaier C, Hertting G, von Kiigelgen O (1987) The adenosine receptor-mediated inhibition of noradrenaline release possibly involves a N-protein and is increased by α2-autoreceptor blockade. Br J Pharmacol 90:403–412
Bailey SL Hourani SMO (1990) A study of the purinoceptors mediating contraction in the rat colon. Br J Pharmacol 100:753–756
Bruns RF (1980) Adenosine receptor activation by adenine nucleotides requires conversion of the nucleotides to adenosine. Naunyn-Schmiedeberg's Arch Pharmacol 315:5–13
Bruns RF, Lu GH, Pugsley TA (1986) Characterization of A2 adenosine receptor labeled by [3H]NECA in rat striatal membranes. Mol Pharmacol 29: 331–346
Bruns RF, Fergus JH, Badger EW, Bristol JA, Santay LA, Hartman JD, Hays SJ, Huang CC (1987) Binding of the A2-selective adenosine antagonist 8-cyclopentyl-1,3-dipropylxanthine to rat brain membranes. Naunyn-Schmiedeberg's Arch Pharmacol 335:59–63
Clanachan AS, Johns A, Paton DM (1977) Presynaptic inhibitory actions of adenine nucleotides and adenosine on neurotransmission in the rat vas deferens. Neuroscience 2:597–602
Collis MG, Pettinger SJ (1982) Can ATP stimulate P1-receptors in guinea-pig atrium without conversion to adenosine? Eur J Pharmacol 81:521–529
De Mey J, Burnstock G, Vanhoutte PM (1979) Modulation of the evoked release of noradrenaline in canine saphenous vein via presynaptic receptors for adenosine but not ATP. Eur J Pharmacol 55:401–405
Deckert J, Morgan PF, Marangos PJ (1988) Adenosine uptake site heterogeneity in the mammalian CNS? Uptake inhibitors as probes and potential neuropharmaceuticals. Life Sci 42:1331–1345
Dunn PM, Blakeley AGH (1988) Suramin: a reversible P2-purinoceptor antagonist in the mouse vas deferens. Br J Pharmacol 93:243–245
Enero MA, Saidman BQ (1977) Possible feed-back inhibition of noradrenaline release by purine compounds. Naunyn-Schmiedeberg's Arch Pharmacol 297:39–46
Feuerstein TJ, Hertting G, Jackisch R (1985) Modulation of hippocampal serotonin (5-HT) release by endogenous adenosine. Eur J Pharmacol 107:233–242
Forsyth KM, Bjur RA, Westfall DP (1991) Nucleotide modulation of norepinephrine release from sympathetic nerves in the rat vas deferens. J Pharmacol Exp Ther 256:821–826
Fredholm BB (1974) Vascular and metabolic effects of theophylline, dibuturyl cyclic AMP and dibuturyl cyclic GMP in canine subcutaneous adipose tissue in situ. Acta Physiol Scand 90:226–236
Fredholm BB, Hedqvist P (1980) Modulation of neurotransmission by purine nucleotides and nucleosides. Biochem Pharmacol 29:1635–1643
Harms HH, Wardeh G, Mulder AH (1978) Adenosine modulates depolarization-induced release of 3H-noradrenaline from slices of rat brain neocortex. Eur J Pharmacol 49: 305–308
Hendley ED, Whittemore SR, Chaffee JE, Ehrlich YH (1988) Regulation of norepinephrine uptake by adenine nucleotides and divalent cations: role for extracellular protein phosphorylation. J Neurochem 50:263–273
Hollins C, Stone TW (1980) Adenosine inhibition of γ-aminobutyric acid release from slices of rat cerebral cortex. Br J Pharmacol 69:107–112
Hourani SMO, Bailey SJ, Nicholls J, Kitchen I (1991) Direct effects of adenylyl 5′-(\,β-methylene)diphosphonate, a stable ATP analogue, on relaxant PI-purinoceptors in smooth muscle. Br J Pharmacol 104:685–690
Hoyle CHV, Burnstock G (1991) ATP receptors and their physiological roles. In: Stone TW (ed.) Adenosine in the nervous system. Academic Press, London, pp 43–76
Illes P, Jackisch R, Regenold JT (1988) Presynaptic P1-purinoceptors in jejunal branches of the rabbit mesenteric artery and their possible function. J Physiol 397:13–29
Jackisch R, Strittmatter H, Kasakov L, Hertting G (1984) Endogenous adenosine as a modulator of hippocampal acetylcholine release. Naunyn-Schmiedeberg's Arch Pharmacol 327:319–325
Jackisch R, Fehr R, Hertting G (1985) Adenosine: an endogenous modulator of hippocampal noradrenaline release. Neuropharmacology 24:499–507
Jonzon B, Fredholm BB (1984) Adenosine receptor mediated inhibition of noradrenaline release from slices of the rat hippocampus. Life Sci 35:1971–1979
Kennedy C (1990) Pl- and P2-purinoceptor subtypes — an update. Arch Int Pharmacodyn Ther 303:30–50
Limberger N, Spath L, Starke K (1988) Presynaptic α2-adrenoceptor, opioid κ-receptor and adenosine Al-receptor interactions on noradrenaline release in rabbit brain cortex. Naunyn-Schmiedeberg's Arch Pharmacol 338:53–61
Lohse MJ, Klotz KN, Lindeborn-Fotinos J, Reddington M, Schwabe U, Olsson RA (1987) 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) — a selective high affinity antagonist radioligand for A1 adenosine receptors. Naunyn-Schmiedeberg's Arch Pharmacol 336:204–210
Lukacsko P, Blumberg A (1982) Modulation of the vasoconstrictor response to adrenergic stimulation by nucleosides and nucleotides. J Pharmacol Exp Ther 222:344–349
Mayer A, Limberger N, Starke K (1988) Transmitter release patterns of noradrenergic, doparninergic and cholinergic axons in rabbit brain slices during short pulse trains, and the operation of presynaptic autoreceptors. Naunyn-Schmiedeberg's Arch Pharmacol 338:632–643
Moody CJ, Meghji P, Burnstock G (1984) Stimulation of P1-purinoceptors by ATP depends partly on its conversion to AMP and adenosine and partly on direct action. Eur J Pharmacol 97:47–54
Pedata F, Antonelli T, Lambertini L, Beani L, Pepeu G (1983) Effect of adenosine, adenosine triphosphate, adenosine deaminase, dipyridamole and aminophylline on acetylcholine release from electrically-stimulated brain slices. Neupharmacology 22: 609–614
Reichenbacher D, Reimann W, Starke K (1982) α-Adrenoceptor-mediated inhibition of noradrenaline release in rabbit brain cortex slices. Naunyn-Schmiedeberg's Arch Pharmacol 319: 71–77
Rice WR, Singleton FM (1987) P2Y-purinoceptor regulation of surfactant secretion from rat isolated alveolar type II cells is associated with mobilization of intracellular calcium. Br J Pharmacol 91:833–838
Richardson PJ, Brown SJ, Bailyes EM, Luzio JP (1987) Ectoenzymes control adenosine modulation of immunoisolated cholinergic synapses. Nature 327:232–234
Shinozuka K, Bjur RA, Westfall DP (1988) Characterization of prejunctional purinoceptors on adrenergic nerves of the rat caudal artery. Naunyn-Schmiedeberg's Arch Pharmacol 338:221–227
Shinozuka K, Bjur RA, Westfall DP (1990) Effects of a,Q-methylene ATP on the prejunctional purinoceptors of the sympathetic nerves of the rat caudal artery. J Pharmacol Exp Ther 254:900–904
Singer EA (1988) Transmitter release from brain slices elicited by single pulses: a powerful method to study presynaptic mechanisms. Trends Pharmacol Sci 9:274–276
Sperlagh B, Vizi ES (1991) Effect of presynaptic P2 receptor stimulation on transmitter release. J Neurochem 56:1466–1470
Starke K (1987) Presynaptic a-autoreceptors. Rev Physiol Biochem Pharmacol 107:73–146
Starke K, Göthert M, Kilbinger H (1989) Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol Rev 69:864–989
Stjärne L, Astrand P (1985) Relative pre- and postjunctional roles of noradrenaline and adenosine 5′-triphosphate as neurotransmitters of the sympathetic nerves of guinea-pig and mouse vas deferens. Neuroscience 14:929–946
Stone TW (1981) Physiological roles for adenosine and adenosine 5′-triphosphate in the nervous system. Neuroscience 6:523–555
Tschöpl M, Harms L, Nörenberg W, Forster E, Illes P (1990) Effects of P2-purinoceptor agonists on peripheral and central noradrenergic neurones. Naunyn-Schmiedeberg's Arch Pharmacol 342: R44
Vizi ES, Knoll J (1976) The inhibitory effect of adenosine and related nucleotides on the release of acetylcholine. Neuroscience 1:391–398
von Kügelgen I, Starke K (1991) Noradrenaline-ATP co-transmission in the sympathetic nervous system. Trends Pharmacol S612:319–324
von Kügelgen I, Schöffel E, Starke K (1989) Inhibition by nuleotides acting at presynaptic P2-receptors of sympathetic neuro-effector transmission in the mouse isolated vas deferens. Naunyn-Schmiedeberg's Arch Pharmacol 340:522–532
Welford LA, Cusack NJ, Hourani SMO (1987) The structure-activity relationships of ectonucleotidases and of excitatory P2-purinoceptors: evident that dephosphorylation of ATP analogues reduces pharmacological potency. Eur J Pharmacol 141:123–130
Wiklund NP, Gustafsson LE, Lundin J (1985) Pre- and postjunctional modulation of cholinergic neuroeffector transmission by adenine nucleotides. Experiments with agonist and antagonist. Acta Physiol Scand 125:681–691
Yount RG (1975) ATP analogs. Adv Enzymol 43:1–56
Author information
Authors and Affiliations
Additional information
Send offprint requests to I. von Kugelgen at the above address
Rights and permissions
About this article
Cite this article
von Kügelgen, I., Späth, L. & Starke, K. Stable adenine nucleotides inhibit [3H]-noradrenaline release in rabbit brain cortex slices by direct action at presynaptic adenosine A1-receptors. Naunyn-Schmiedeberg's Arch Pharmacol 346, 187–196 (1992). https://doi.org/10.1007/BF00165300
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00165300