Abstract
To investigate the value of the extrafoveal pattern electroretinogram for evaluation of local retinal defects, electroretinographic responses to contrast reversal stimulation were recorded at various locations in the central and midperipheral retina. Normal values were established in 20 eyes, and spatial selectivity at different retinal eccentricities was determined in four eyes.
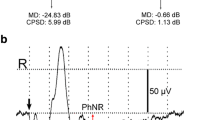
The response amplitude was found to decrease steeply from the fovea until about 12° eccentricity and to decline only slowly thereafter. For central stimulation a clear attenuation for coarser patterns was observed. At 20° and at 30° eccentricity the spatial tuning function exhibited a bandpass characteristic, with the maximum amplitude shifting to lower spatial frequencies. Despite the relatively low peripheral amplitudes a satisfactory signal to noise ratio of the second harmonic responses can be obtained by Fourier analysis, which improves clinical applicability. The comparison of sensitivity in the upper and lower retina revealed a considerable asymmetry, with responses in the upper retina approximately 20% higher. No significant difference, however, was found when the temporal or nasal retina was stimulated. The reproducibility of these results is high enough to encourage clinical studies. A case of retinal venous branch occlusion exemplifies the applicability of this technique in eccentric fundus lesions.
Similar content being viewed by others
References
Celesia GG, Kaufman D. Pattern ERGs and visual evoked potentials in maculopathies and optic nerve diseases. Invest Ophthalmol Vis Sci 1985; 26: 726–35.
Wanger P, Persson H. Pattern-reversal electroretinograms in ocular hypertension. Doc Ophthalmol 1985; 61: 27–31.
Arden GB, Hamilton AMP, Wilson-Holt J, Ryan S, Yudkin JS, Kurtz A. Pattern electroretinograms become abnormal when background retinopathy deteriorates to a preproliferative stage: possible use as a screening test. Br J Ophthalmol 1986; 70: 330–5.
Dodt E. The electrical response of the human eye to patterned stimuli: Clinical observations. Doc Ophthalmol 1987; 65: 271–86.
Zrenner E, Ziegler R, Voss B. Clinical applications of pattern electroretinography: Melanoma, retinal detachment and glaucoma. Doc Ophthalmol 1988; 68: 283–92.
Lorenz R, Niepel G, Heider W. Amplituden- und Latenzänderungen im Muster-Elektroretinogramm (M-ERG) bei Netzhaut- und Sehnervenerkrankungen. Fortschr Ophthalmol 1988; 85: 296–300.
Riggs LA, Johnson EP, Schick AML. Electrical responses of the human eye to moving stimulus patterns. Science 1964; 144: 567.
Groneberg A, Teping C. Topodiagnostik von Sehstörungen nach Ableitung retinaler und kortikaler Antworten auf Umkehr-Kontrastmuster. Ber Dtsch Ophthalmol Ges 1980; 77: 409–15.
Arden GB, Vaegan Hogg CR. Clinical and experimental evidence that the pattern electroretinogram (PERG) is generated in more proximal retinal layers than the focal electroretinogram (FERG). Ann NY Acad Sci 1982; 79: 580–601.
Van Lith G, Ringens P, de Heer LJ. Pattern electroretinogram and glaucoma. Dev Ophthalmol 1984; 9: 133–9.
Maffei L, Fiorentini A. Electroretinographic responses to alternating gratings in the cat. Exp Brain Res 1982; 48: 327–34.
Maffei L, Fiorentini A, Bisti S, Holländer H. Pattern ERG in the monkey after section of the optic nerve. Ex Brain Res 1985; 59: 423–5.
Sieving PA, Steinberg RH. Current source density analysis of intraretinal 8 Hz pattern ERG identifies contribution from proximal retina in cat. J Neurophysiol 1987; 57: 104–20.
Zrenner E, Baker CL Jr, Hess RF, Olsen BT. Die Lokalisation elektroretinographischer Antworten auf Musterum Kehrreize und Helligkeitsreize in einzelnen Schichten der Primatennetzhaut. Fortschr Ophthalmol 1987; 84: 491–5.
Baker CL Jr, Hess RF, Zrenner E, Olsen BT. Current source density analysis of linear and nonlinear components of the primate electroretinogram. J Physiol 1988; 407: 155–76.
Zimmerman LE. Embolism of central retinal artery. Arch Ophthalmol 1965; 73: 822–6.
Daicker B. Pathologische Anatomie der arteriellen und venösen Gefäßverschlüsse in der Netzhaut. Klin Monatsbl Augenheilkd 1977; 170: 198–211.
Vomberg HE, Heider W. Muster- und Helligkeits-Elektroretinogramm bei Verschlüssen von Netzhautgefäßen. Klin Monatsbl Augenheilkd 1985; 187: 503–7.
Hess RF, Baker CL Jr. Human pattern-evoked electroretinogram. J Neurophysiol 1984; 51: 939–51.
Arden GB, Carter RM, Hogg C, Siegel IM, Margolis S. A gold foil electrode: extending the horizons for clinical electroretinography. Invest Ophthalmol Vis Sci 1979; 18: 421–6.
Perry VH, Cowey A. The ganglion cell and cone distribution in the monkey's retina: implications for central magnification factors. Vision Res 1985; 1795–810.
Ranson-Hogg A, Spillmann L. Perceptive field size in fovea and periphery of the lightand dark-adapted retina. Vision Res 1980; 20: 221–8.
Skrandies W. Scalp potential fields evoked by grating stimuli: effects of spatial frequency and orientation. Electroencephalogr Clin Neurophysiol 1984; 58: 325–32.
Yoshii M, Päärmann A. Half-field stimuli elicit differences in pattern ERG responses. Doc Ophthalmol 1988; (in press).
Perry VH, Oehler R, Cowey A. Retinal ganglion cells that project to the dorsal lateral geniculate nucleus in the macaque monkey. Neuroscience 1984; 12: 1101–23.
Skrandies W, Baier M. The standing potential of the human eye reflects differences between upper and lower retinal areas. Vision Res 1986; 26: 577–81.
Lehmann D, Skrandies W, Multichannel evoked potential fields show different properties of human upper and lower hemiretina systems. Exp Brain Res 1979; 35: 151–9.
Skrandies W. Human contrast sensitivity: regional retinal differences. Hum Neurobiol 1979; 4: 97–9.
Skrandies W. Critical flicker fusion and double flash discrimination in different parts of the visual field. Int J Neurosci 1988; 25: 225–31.
Bopp M. Predominance of the nasal hemiretina in the pattern electroretinogram of the human eye. Pflügers Arch Suppl 1982; 392: R50.
Fahle M, Schmid M. Naso-temporal asymmetry of visual perception. Vision Res 1982; 28: 293–300.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Marx, R., Zrenner, E. Sensitivity distribution in the central and midperipheral visual field determined by pattern electroretinography and harmonic analysis. Doc Ophthalmol 73, 347–357 (1989). https://doi.org/10.1007/BF00154490
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00154490