Summary
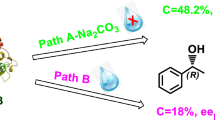
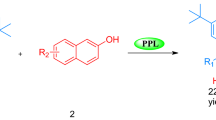
n-Alkyl sec-alkyl carbonates were enantioselectively hydrolyzed by porcine pancreatic lipase to give optically active (R)-sec-alkanols. (R)-1-Phenylethanol with an optical purity of >99%ee was obtained by the resolving method.
Similar content being viewed by others
References
Boland, W., Fröβl, C., and Lorenz, M. (1991). Synthesis 1049–1072.
Naoshima, Y., Munakata, Y., Yoshida, S., and Funai, A. (1991). J. Chem. Soc., Perkin Trans 1 549–553.
Abramowicz, D. A. and Keese, C. R. (1989). Biotechnol. Bioeng. 33, 149–156.
Andreoni, V., Baggi, G., Bernasconi, S., Foglieni, C., and Pelizzoni, F. (1990). Appl. Microbiol. Biotechnol. 33, 633–636.
Kawashima, M. and Hasegawa, T. (1992). Biotechnol. Lett. 14, 1135–1136.
Chen, C. -S., Fujimoto, Y., Girdaukas, G., and Sih, C. J. (1982). J. Am. Chem. Soc. 104, 7294–7299.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kawashima, M., Hasegawa, T. First enantioselective hydrolysis of n-alkyl sec-alkyl carbonates by porcine pancreatic lipase. Biotechnol Lett 15, 465–468 (1993). https://doi.org/10.1007/BF00129319
Issue Date:
DOI: https://doi.org/10.1007/BF00129319