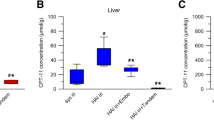
Systemic treatment with tumor necrosis factor (TNF) is associated with side-effects, limiting its clinical use in the treatment of malignancies. To investigate the feasibility of other routes of administration experimental and clinical studies were started to establish the toxicity and antitumor activity of TNF after intratumoral (i.t.) injection. In a rat model for colon adenocarcinoma, tumor fragments, implanted subcutaneously or under the hepatic capsule, were treated with TNF injected i.v. or i.t. A dosage of 40 μg/kg was lethal when given i.v., but not i.t. Injection of TNF (40 μg/kg) directly into the tumor resulted in inhibition of tumor growth in the subcutaneous as well as subhepatic tumor model. A phase I study was started in patients with advanced malignancies to determine the toxicity of TNF injected into liver metastases. Injection of TNF into liver metastases was accomplished by ultrasonography. A 50 μg-dose escalating schedule (3 patients/dosage) was chosen, starting at a dose of 100 μg TNF/injection. Up to now, 12 patients have been treated, the highest dosage of TNF injected being 250 μg. Chills, fever, nausea and vomiting were the main side-effects. No significant changes were found in circulatory, hematologic, renal and liver parameters. In summary, i.t. administration of TNF is associated with antitumor efficacy in experimental models and well-tolerated in man. The antitumor efficacy of TNF i.t. in man awaits evaluation in a phase II study.
Similar content being viewed by others
References
Balkwill FR, 1989, Tumour necrosis factor. British Medical Bulletin, 45, 389–400.
Mannel DN, Moore RN and Mergenhagen SE, 1980, Macrophages as a source of tumoricidal activity (tumor- necrotizing factor). Infections and Immunity, 30, 523–530.
O'Malley WE, Achinstein B and Shear MH, 1962, Action of bacterial polysaccharide on tumours 11. Damage of sarcoma 37 by serum of mice treated with Serratia marcescens polysaccharide, and induced tolerance. JNCI, 29, 1169–1175.
Pennica D, Nedwin GE, Hayflick JS, Seeburg PH, Derynck R, Palladino MA, Kohr WJ, Aggarwal BB and Goeddel DV, 1984, Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature, 312, 724–729.
Collins T, Lapierre LA, Fiers W, Strominger JL and Pober JS, 1986, Recombinant human tumor necrosis factor increases mRNA levels and surface expression of HLA-A, B antigens in vascular endothelial cells and dermal fibroblasts in vitro. Proceedings of the National Academy of Sciences USA, 83, 446–450.
Dinarello CA, Cannon JG, Wolff SM, Bernheim HA, Beutler B, Cerami A, Figari IS, Palladino MA Jr and O'Connor JV, 1986, Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin-1. Journal of Experimental Medicine, 163, 1433–1450.
Marquet RL, Westbroek DL and Jeekel J, 1984, Interferon treatment of a transplantable colon adenocarcinoma; importance of tumor site. International Journal of Cancer, 33, 689–692.
Torti FM, Dieckmann B, Beutler B, Cerami A and Ringold GM, 1985, A macrophage factor inhibits adipocyte gene expression: an in vitro model of cachexia. Science, 229, 867–869.
Beutler BA and Cerami AC, 1986, Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature, 320, 584–588.
Tracey KJ, Lowry SF and Fahey TJ, 1987, Cachectin/tumor necrosis factor induces lethal shock and stress hormone responses in the dog. Surgical Gynecology and Obstetrics, 164, 415–421.
Aggarwal BB, Eessalu TE and Hass PE, 1985, Characterization of receptors for human tumour necrosis factor and their regulation by gamma interferon. Nature, 318, 665–666.
Bevilacqua MP, Pober JS, Majeau GR, Fiers W, Cotran RS and Gimbrone MA, Jr, 1986, Recombinant tumor necrosis factor induces procoagulant activity in cultured human vascular endothelium: characterization and comparison with the actions of interleukin-1. Proceedings of the National Academy of Sciences USA, 83, 4533–4537.
Nawroth PP and Stern DM, 1986, Modulation of endothelial cell hemostatic properties by tumor necrosis factor. Journal of Experimental Methods, 163, 740–745.
Sato N, Goto T, Harnaka K, Satomi N, Nariuchi H, Mano-Hirano Y and Sawasaki Y, 1986, Actions of tumor necrosis factor on cultured vascular endothelial cells: morphologic modulation, growth inhibition, and cytotoxicity. JNCI, 76, 1113–1121.
Nawroth PP, Bank I, Handley D, Cassimeris J, Chess L and Stern D, 1986, Tumor necrosis factor/ cachectin interacts with endothelial cell receptors to induce release of interleukin-1. Journal of the Experimental Methods, 163, 1363–1375.
Prehn RT and Main JW, 1957, Immunity to methylcholanthrene induced sarcomas. Journal of the National Cancer Institute, 18, 769–778.
Fransen L, Muller R, Marmenout A, Tavenier J, van der Heyden J, Kawashima E, Chollet A, Tizard R, van Heuverswyn H, van Vliet A, Ruysschaert M and Fiers W, 1985, Molecular cloning of mouse tumor necrosis factor cDNA and its eukaryotic expression. Nucleic Acids Research, 13, 4417–4429.
Marquet RL, Uzermans JNM, deBruin RWF, Fiers W and Jeekel J, 1987, Antitumor activity of recombinant mouse tumor necrosis factor (TNF) on colon cancer in rats is promoted by recombinant rat interferon-gamma; toxicity is reduced by indomethacine. International Journal of Cancer, 40, 550–553.
Fiers W, Brouckeart P, Guiser Y, Remaut E, van-Roy F, Devos R, Fransen L, Leroux-Roels G, Marmenout A, Tavernier J and van der Heyden J. Recombinant interferon gamma and its synergism with tumor necrosis factor in the human and mouse systems. In: Stewart WE and Schellekens H, eds. The Biology of the Interferon System. London: Elsevier Science Publishers, 1986, pp. 112–120.
Haranaka K, Satomi N and Sakurai A, 1984, Antitumor activity of murine tumor necrosis factor (TNF) against transplanted murine tumors and het erotransplanted human tumors in nude mice. International Journal of Cancer, 34, 263–267.
Havell EA, Fiers W and North RJ, 1988, The antitumor function of tumor necrosis factor (TNF) 1. Journal of Experimental Methods, 167, 1067–1085.
Rosenberg SA, 1986, The adoptive immunotherapy of cancer using the transfer of activated lymphoid cells and interleukin-2. Seminars in Oncology, 13, 200–206.
Vadhan-raj S, Nathan C, Bhalla R, Pelus L, Nathan CF, Sherwin SA, Oettgen HF and Krown SE, 1986, Phase I trial of recombinant interferon gamma by six hours intravenous infusion. J. Clin. Oncol., 4, 137–14624.
Creaven PJ, Benner DE, Cowens JW, Huben RP, Wof RM, Takita H, Arbuck SG, Razack MS and Proefrock AD, 1989, A Phase I clincal trial of recombinant tumor necrosis factor given daily for five days. Cancer Chemotherapy and Pharmacology, 23, 186–189.
Steinmetz T, Schaadt M, Gahl R, Schenk V, Diehl V and Pfreundschuh M, 1988, Phase I study of 24-hour continuous infusion of recombinant human tumor necrosis factor. Journal of Biological Response Modifiers, 7, 417–423.
Bartsch HH, Pfizenmaier K, Schroeder M and Nagel GA, 1989, Intralesional application of recombinant human tumor necrosis factor alpha induces local tumor regression in patients with advanced malignancies. European Journal of Cancer and Clinical Oncology, 25, 287–291.
Pfreundschuh MG, Steinmetz HT, Tueschen R, Schenk V, Diehl V and Schaadt M, 1989, Phase I study of intratumoral application of recombinant human tumor necrosis factor. European Journal of Cancer and Clinical Oncology, 25, 379–388.
Kahn JO, Kaplan LD, Volberding PA, Ziegler JL, Crowe S, Saks Sr and Abrams DI, 1989, Intralesional recombinant tumor necrosis factor-alpha for AIDS-associated Kaposi's sarcoma: a randomized, double-blind trial. Journal of Acquired Immune Deficiency Syndrome, 2, 217–223.
Munker R, Gasson J, Ogawa M and Koeffler HP, 1986, Recombinant human TNF induces production of granulocyte-monocyte colony-stimulating factor. Nature, 323, 79–82.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
IJzermans, J.N.M., Scheringa, M., van der Schelling, G.P. et al. Injection of recombinant tumor necrosis factor directly into liver metastases: an experimental and clinical approach. Clin Exp Metast 10, 91–97 (1992). https://doi.org/10.1007/BF00114585
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00114585