Abstract
Decomposition rates, determined with the litterbag technique in salt marshes of the S.W. Netherlands during the past decade are compared; the biotic and abiotic factors influencing these rates are identified and discussed.
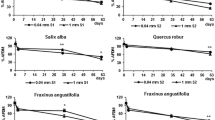
Tissue composition is the main variable affecting decay rates of halophytes, particularly variations in lignin content between plant parts and between species.
Experiments in which the loss of the tensile strength of cotton strips was used as an index of cellulolytic decay, show that there is a conspicuous variation in decay rates on different sites in a salt marsh. Nonetheless, the locally varying environmental conditions within salt marshes of the S.W. Netherlands have less impact on the variation in decomposition rates of halophyte litter than the chemical make-up of the plant material.
Larger fauna elements (> 300 μm) may increase decomposition rates, but this effect is only limited and depends on location and litter type. The role of small fauna elements such as nematodes, which occur abundantly in association with halophyte litter, remains largely unknown.
Similar content being viewed by others
References
Abrams, B. I. & Mitchell, M. J. 1980. Role of nematode-bacterial interactions in heterotrophic systems with emphasis on sewage sludge decomposition. Oikos 35: 404–440.
Ambrose, W. R. 1980. Monitoring long-term temperature and humidity. Bull. Inst. Conserv. Cult. Mater. 6: 36–42.
Armstrong, W., Wright, E. J., Lythe, S. & Gaynard, T. J. 1980. Plant zonation and the effects of the spring-neap tidal cycle on soil aeration in a Humber salt marsh. J. Ecol. 73: 323–339.
Beeftink, W. G. 1977. The coastal salt marshes of Western and Northern Europe: an ecological and phytosociological approach. In: Chapman, V. J. (ed.) Wet coastal ecosystems. Elseviers, Amsterdam, p. 109–155.
Buth, G. J. C. 1987. Decomposition of roots of three plant communities in a Dutch salt marsh. Aquat. Bot. 29: 123–138.
Buth, G. J. C., Verdonschot, P. F. M. & DeWolf, L. 1982. Decomposition of three halophytes in different habitats of an Eastern Scheldt salt marsh. Hydrobiol. Bull. 16: 103–112.
Buth, G. J. C. & Voesenek, L. A. C. J. 1987. Decomposition of standing and fallen litter of halophytes in a Dutch salt marsh. In: Huiskes, A. H. L., Blom, C. W. P. M. & Rozema, J. (eds.) Vegetation between land and sea. Junk, Dordrecht, p. 146–162.
Buth, G. J. C. & Voesenek, L. A. C. J. 1988. Respiration of standing and fallen plant litter in a Dutch salt marsh. In: Verhoeven, J. T. A., Heil, G. W. & Werger, M. J. A. (Eds.) Vegetation structure in relation to carbon and nutrient economy. SPB Academic Publ., Den Haag, p. 51–60.
Buth, G. J. C. & DeWolf, L. 1985. Decomposition of Spartina anglica, Elytrigia pungens and Halimione portulacoides in a Dutch salt marsh in association with faunal and habitat influences. Vegetatio: 62: 337–355.
Christian, R. R. 1984. A life-table approach to decomposition studies. Ecology 65: 1693–1697.
Clarholm, M. 1985. Interactions of bacteria, protozoa and plants leading to mineralization of soil nitrogen. Soil. Biol. Biochem. 17: 181–187.
Coull, B. C. & Bell, S. S. 1979. Perspectives of marine meiofaunal ecology. In: R. J.Livingstone (Ed.). Ecological Processes in Coastal and Marine Systems. Plenum Press, New York, p. 189–216.
Cowling, E. B. & Brown, W. 1969. Structural features of cellulosic materials in relation to enzymatic hydrolysis. In: Hajny, G. J., Reese, E. T. (Ed.). Cellulases and their applications. Adv. Chem. Ser. Publ. No. 95, Amer. Chem. Soc., Washington, DC.
De laCruz, A. A. & Hackney, C. T. 1977. Energy value, elemental composition, and productivity of below-ground biomass of a Juncus tidal marsh. Ecology 58: 1165–1170.
Findlay, S. & Tenore, K. 1982. Effect of a free-living marine nematode (Diplolaimella chitwoodie) on detrital carbon mineralization. Mar. Ecol. Prog. Ser. 8: 161–166.
French, D. O. 1988. Some effects of changing soil chemistry on decomposition of plant litter and cellulose on a Scottish moor. Oecologia 75: 608–618.
Gallagher, J. L. & Plumley, F. G. 1979. Underground biomass profiles and productivity in Atlantic coastal marshes. Am. J. Bot. 66: 156–161.
Gallagher, J. L., Kibby, H. V. & Skirvin, K. W. 1984. Community respiration of decomposing plants in Oregon Estuarine Marshes. Est. coastal Sh. Sci. 18: 421–431.
Hackney, C. T. 1987. Factors affecting accumulation or loss of macroorganic matter in salt marsh sediments. Ecology 68: 1109–1113.
Harvey, P. J., Schoemaker, H. E. & Palmer, J. M. 1987. Lignin degradation by white rot fungi. Plant, Cell Environm. 10: 709–714.
Hemminga, M. A., Kok, C. J. & DeMunck, W. 1988. Decomposition of Spartina anglica roots and rhizomes in a salt marsh of the Westerschelde estuary. Mar. Ecol. Prog. Ser. 48: 175–184.
Gildrew, A. G., Townsend, C. R., Francis, J. & Fench, K. 1984. Cellulolytic decomposition in streams of contrasting pH and its relationship with invertebrate community structure. Freshw. Biol. 14: 323–328.
Hill, M. O., Latter, P. M. & Bancroft, G. 1985. A standard curve for intersite comparison of cellulose degradation using the cottong strip method. Can. J. Soil. Sci. 65: 609–619.
Kemp, P. F., Newell, S. Y. & Hopkinson, C. S. 1990. Importance of grazing on the salt-marsh grass Spartina alterniflora to nitrogen turnover in a macrofaunal consumer, Littorina irrorata, and to decomposition of standing-dead Spartina. Mar. Biol. 104: 311–319.
Livingstone, D. C. & Patriquin, D. G. 1981. Belowground growth of Spartina alterniflora Loisel: Habitats, functional biomass and non-structural carbohydrates. Est. coastal. Shelf Sci. 12: 579–587.
Lopez, G. R., Levinton, J. S. & Slobodkin, L. B. 1977. The effect of grazing by the detritivore Orchestia grillus on Spartina litter and its associated microbial community. Oecologia 30: 111–127.
Lynch, J. M. 1976. Products of soil microorganisms in relation to plant growth. Crit. Rev. Microbiol. 5: 67–107.
Marinucci, A. C., Hobbie, J. E. & Helfrich, J. V. K. 1983. Effect of litter nitrogen on decomposition and microbial biomass in Spartina alterniflora. Microbiol. Ecol. 9: 27–40.
Newell, S. Y., Fallon, R. D., Rodriguez, R. M. C. & Groene, L. C. 1985. Influence of rain, tidal wetting and relative humidity on release of carbon dioxide by standing-dead salt-marsh plants. Oecologia 68: 73–79.
Reice, S. R. & Stiven, A. E. 1983. Environmental patchiness, litter decomposition and associated faunal patterns in a Spartina alterniflora marsh. Est. coast. Shelf. Sci. 16: 559–571.
Reimold, R. J. 1977. Mangals and salt marshes of Eastern United States. In: Chapman, V. J. (Ed.) Wet coastal ecosystems. Elseviers, Amsterdam, p. 157–166.
Smith, K. K., Good, R. E. & Wood, N. F. 1979. Production dynamics for above and below ground components of a New Jersey Spartina alterniflora tidal marsh. Est. coast. mar. Sci. 9: 189–201.
Swift, M. J., Heal, O. W. & Anderson, J. M. 1979. Decomposition in terrestrial ecosystems. Blackwell, Oxford.
Valiela, I., Teal, J. M., Allen, S. E., VanEtten, R., Goehringer, D. & Volkman, S. 1985. Decomposition in salt marsh ecosystems: the phases and major factors affecting disappearance of above-ground organic matter. J. exp. mar. Biol. Ecol. 89: 29–54.
White, D. A. & Trapani, J. M. 1982. Factors influencing disappearance of Spartina alterniflora from litterbags. Ecology 63: 242–245.
Wieser, W. 1959. Fee-living nematodes. IV. General part. Reports of the Lund University expedition 1948–1949. Lunds Universiteits Arsskrift. N.F. 2.55,5 Hakan Ohlssons, Lund, p. 68–85.
Wilson, J. O., Buchsbaum, R., Valiela, I. & Swain, T. 1986a. Decomposition in salt marsh ecosystems: phenolic dynamics during decay of litter of Spartina alterniflora. Mar. Ecol. Prog. Ser. 29: 177–187.
Wilson, J. O., Valiela, I. & Swain, T. 1986b. Carbohydrate dynamics during decay of litter of Spartina alterniflora. Mar. Biol. 92: 277–284.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hemminga, M.A., Buth, G.J.C. Decomposition in salt marsh ecosystems of the S.W. Netherlands: the effects of biotic and abiotic factors. Vegetatio 92, 73–83 (1991). https://doi.org/10.1007/BF00047133
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00047133