Abstract
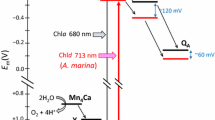
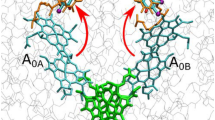
The photooxidation of the primary electron donor in several Photosystem I-related organisms (Synechocystis sp. PCC 6803, Heliobacillus mobilis, and Chlorobium limicola f. sp. thiosulphatophilum) has been studied by light-induced FTIR difference spectroscopy at 100 K in the 4000 to 1200 cm−1 spectral range. The data are compared to the well-characterized FTIR difference spectra of the photooxidation of the primary donor P in Rhodobacter sphaeroides (both wild type and the heterodimer mutant HL M202) in order to get information on the charge localization and the extent of coupling within the (bacterio)chlorophylls constituting the oxidized primary donors. In Rb. sphaeroides RC, four marker bands mostly related to the dimeric nature of the oxidized primary donor have been previously observed at ≈2600, 1550, 1480, and 1295 cm−1. The high-frequency band has been shown to correspond to an electronic transition (Breton et al. (1992) Biochemistry 31: 7503–7510) while the three other marker bands have been described as phase-phonon bands (Reimers and Hush (1995) Chem Phys 197: 323–332). The absence of these bands in PS I as well as in the heterodimer HL M202 demonstrates that in P700+ the charge is essentially localized on a single chlorophyll molecule. For both H. mobilis and C. limicola, the presence of a high-frequency band at ≈ 2050 and 2450 cm−1, respectively, and of phase-phonon bands (at ≈ 1535 and 1300 cm−1 in H. mobilis, at ≈ 1465 and 1280 cm-1 in C. limicola) indicate that the positive charge in the photooxidized primary donor is shared between two coupled BChls. The structure of P840+ in C. limicola, in terms of the resonance interactions between the two BChl a molecules constituting the oxidized primary donor, is close to that of P+ in purple bacteria reaction centers while for H. mobilis the FTIR data are interpreted in terms of a weaker coupling of the two bacteriochlorophylls.
Similar content being viewed by others
Abbreviations
- (B)Chl:
-
(bacterio)chlorophyll
- BPhe:
-
bacteriopheophytin
- C. :
-
Chlorobium
- FTIR:
-
Fourier transform infrared
- H. :
-
Heliobacillus
- PS I, PS II:
-
Photosystem I, Photosystem II
- P:
-
primary electron donor
- RC:
-
reaction center
- Rb. :
-
Rhodobacter
- Rp. :
-
Rhodopseudomonas
- QA :
-
primary quinone acceptor
- Wt:
-
wild type
References
Albouy D (1995) Etude des protéines impliquées dans les premières étapes de la photosynthèse chez la bactérie verte sulfureuse Chlorobium limicola forma thiosulfatophilum. PhD Thesis, University of Paris XI
Amesz J (1995) The antenna-reaction center complex of heliobacteria. In: Blankenship RE, Madigan MT and Bauer CE (eds) Anoxygenic Photosynthetic Bacteria, pp 687–697. Kluwer Academic Publishers, Dordrecht, The Netherlands
Bauscher M, Mäntele W (1992) Electrochemical and infrared spectroscopic characterization of redox reactions of p-quinones. J. Phys Chem 95: 11101–11108
Binstead RA, Hush NS (1993) Hole localization and spin coupling in π-mono and π-dications of μ-oxoporphyrin dimers. Relevance to structure of oxidized ‘special pair’ in photosynthetic reaction centers. J Phys Chem 97: 13172–13179
Breton J, Nabedryk E and Parson WW (1992) A new infrared electronic transition of the oxidized primary electron donor in bacterial reaction centers: A way to assess resonance interactions between the bacteriochlorophylls. Biochemistry 31: 7503–7510
Breton J, Burie J-R, Berthomieu C, Berger G and Nabedryk E (1994) The binding sites of quinones in photosynthetic bacterial reaction centers investigated by light-induced FTIR difference spectroscopy: Assignment of the QA vibrations in Rhodobacter sphaeroides using 18O-or 13C-labeled ubiquinone and vitamin K1. Biochemistry 33: 4953–4965
Büttner M, Xie D-L, Nelson H, Pinther W, Hauska G and Nelson N (1992) Photosynthetic reaction center genes in green sulfur bacteria and in Photosystem I are related. Proc Natl Acad Sci USA 89: 8135–8139
Cui L, Bingham SE, Kuhn M, Käss H, Lubitz W and Webber AN (1995) Site-directed mutagenesis of conserved histidines in the helix VIII domain of PsaB impairs assembly of the Photosystem I reaction center without altering spectroscopic characteristics of P700+. Biochemistry 34: 1549–1558
Davis D, Dong A, Caughey WS and Schenck CC (1992) Energetics of the oxidized, primary donor in wt and heterodimer mutant reaction centers. Biophys J 61: A153
Davis IH, Heathcote P, MacLachlan DJ and Evans MCW (1993) Modulation analysis of the electron spin echo signals of in vivo oxidised primary donor 14N chlorophyll centres in bacterial, P870 and P960, and plant Photosystem I, P700, reaction centres. Biochim Biophys Acta 1143: 183–189
Evans MCW, Nugent JHA (1993) Structure and function of the reaction center cofactors in oxygenic organisms. In: Deisenhofer J and Norris JR (eds) The Photosynthetic Reaction Center, Vol I, 391–415. Academic Press, San Diego
Feher G (1992) Identification and characterization of the primary donor in bacterial photosynthesis: A chronological account of an EPR/ENDOR investigation. J Chem Soc Perkin Trans 2: 1861–1874
Feiler U and Hauska G (1995) The reaction center from green sulfur bacteria. In: Blankenship RE, Madigan MT and Bauer CE (eds) Anoxygenic Photosynthetic Bacteria, pp 649–663 Kluwer Academic Publishers, Dordrecht, The Netherlands
Feiler U, Albouy D, Robert B and Mattioli TA (1995) Symmetric structural features and binding site of the primary electron donor in the reaction center of Chlorobium. Biochemistry 33: 7594–7599
Fish LE, Kück U and Bogorad (1985) Two partially homologous adjacent light-inducible maize chloroplast genes encoding polypeptides of the P700 chlorophyll a-protein complex of Photosystem I. J Biol Chem 260: 1413–1421
Golbeck JH (1993) The structure of Photosystem I. Curr Opin Struct Biol 3: 508–514
Hamm P and Zinth W (1995) Ultrafast initial reaction in bacterial photosynthesis revealed by femtosecond infrared spectroscopy. J Phys Chem 99: 13537–13544
Hu S and Spiro TG (1993) The origin of infrared marker bands of porphyrin π-cation radicals: Infrared assignments for cations of copper (II) complexes of octaethylporphine and tetraphenylporphine. J Am Chem Soc. 115: 12029–12034
Käss H, Fromme P, Witt HT and Lubitz W (1994) 1H ENDOR and 14N ESEEM of P700+ in single crystals of Photosystem I from Synechococcus elongatus. Biophys J 66: A228
Kleinfeld D, Okamura MY and Feher G (1984) Electron-transfer kinetics in photosynthetic reaction centers cooled to cryogenic temperatures in the charge separated state: Evidence for light-induced structural changes. Biochemistry 23: 5780–5786
Krauss N, Hinrichs W, Witt I, Fromme P, Pritzkow W, Dautez Z, Betzel C, Wilson KS, Witt HT and Saenger W (1993) Three-dimensional structure of system I of photosynthesis at 6 Å resolution. Nature 361: 326–331
Lancaster CRD, Ermler U and Michel H (1995) The structures of photosynthetic reaction centers from purple bacteria as revealed by X-ray crystallography. In: Blankenship RE, Madigan MT and Bauer CE (eds) Anoxygenic Photosynthetic Bacteria, pp 503–526. Kluwer Academic Publishers, Dordrecht, The Netherlands
Lendzian F, Huber M, Isaacson RA, Endeward B, Plato M, Bönigk B, Möbius K, Lubitz W and Feher G (1993) The electronic structure of the primary donor cation radical in Rhodobacter sphaeroides R-26: ENDOR and TRIPLE resonance studies in single crystals of reaction centers. Biochim Biophys Acta 1183: 139–160
Leonhard M, Mäntele W (1993) Fourier transform infrared spectroscopy and electrochemistry of the primary electron donor in Rhodobacter sphaeroides and Rhodopseudomonas viridis reaction centers: Vibrational modes of the pigments in situ and evidence for protein and water modes affected by P+ formation. Biochemistry 32: 4532–4538
Liebl U, Mockensturm-Wilson M, Trost JT, Brune DC, Blankenship RE and Vermaas W (1993) Single core polypeptide in the reaction center of the photosynthetic bacterium Heliobacillus mobilis: Structural implications and relations to other systems. Proc Natl Acad Sci USA 90: 7124–7128
MacDonald GM, Bixby KA and Barry BA (1993) A difference Fourier-transform infrared study of two redox-active tyrosine residues in Photosystem II. Proc Natl Acad Sci USA 90: 11024–11028
Maiti S, Cowen BR, Diller R, Iannone M, Moser CC, Dutton PL and Hochstrasser RM (1993) Picosecond infrared studies of the dynamics of the photosynthetic reaction center. Proc Natl Acad Sci USA 90: 5247–5251
Mäntele W (1993) Infrared vibrational spectroscopy of the photosynthetic reaction center. In: Deisenhofer H and Norris JR (eds) The Photosynthetic Reaction Center, Vol II, pp 239–283. Academic Press, San Diego
Mäntele WG, Wollenweber A, Nabedryk E, Breton J, Rashwan F, Heinze J and Kreutz W (1987) Fourier transform infrared (FTIR) spectroelectrochemistry of bacteriochlorophylls. In: Biggins J (ed) Progress in Photosynthesis Research. Vol I, pp 329–332. Martinus Nijhoff Publishers, Dordrecht, The Netherlands
Mäntele WG, Wollenweber AM, Nabedryk E and Breton J (1988) Infrared spectroelectrochemistry of bacteriochlorophylls and bacteriopheophytins: Implications for the binding of the pigments in the reaction center from photosynthetic bacteria. Proc Natl Acad Sci USA 85: 8468–8472
Michel H and Deisenhofer J (1988) Relevance of the photosynthetic reaction center from purple bacteria to the structure of Photosystem II. Biochemistry 27: 1–7
Nabedryk E (1996) Light-induced Fourier transform infrared difference spectroscopy of the primary electron donor in photosynthetic reaction centers. In: Mantsch HH and Chapman D (eds) Infrared Spectroscopy of Biomolecules, pp 39–81. Wiley-Liss, New York
Nabedryk E, Leonhard M, Mäntele W and Breton J (1990a) Fourier transform infrared difference spectroscopy shows no evidence for an enolization of chlorophyll a upon cation formation either in vitro or during P700 photooxidation. Biochemistry 29: 3242–3247
Nabedryk E, Bagley KA, Thibodeau DL, Bauscher M, Mäntele W and Breton J (1990b) A protein conformational change associated with the photoreduction of the primary and secondary quinones in the bacterial reaction center. FEBS Lett 266: 59–62
Nabedryk E, Robles SJ, Goldman E, Youvan DC and Breton J (1992) Probing the primary donor environment in the histidineM200 → leucine and histidineL173 → leucine heterodimer mutants of Rhodobacter capsulatus by light-induced Fourier transform infrared difference spectroscopy. Biochemistry 31: 10852–10858
Nabedryk E, Allen JP, Taguchi AKW, Williams JC, Woodbury NW and Breton J (1993) Fourier transform infrared study of the primary electron donor in chromatophores of Rhodobacter sphaeroides with reaction centers genetically modified at residues M160 and L131. Biochemistry 32: 13879–13885
Oh-oka H, Kakutani S, Kamei S, Matsubara H, Iwaki M and Itoh S (1995) Highly purified photosynthetic reaction center (PscA/Cytochrome c551)2 complex of the green sulfur bacterium Chlorobium limicola. Biochemistry 34: 13091–13097
Parson WW, Nabedryk E and Breton J (1992) Mid-and near-IR electronic transitions of P+: New probes of resonance interactions and structural asymmetry in reaction centers. In: Breton J and Verméglio A (eds) The Photosynthetic Bacterial Reaction Center II, Structure, Spectroscopy and Dynamics, pp 70–88. Plenum Press, New York
Rautter J, Bönigk B, Lubitz, W, Chiou H-C and Blankenship RE (1996) ENDOR studies of the cation radical of the primary donor in Heliobacteria suggest that P798+ is a symmetric dimer. Biophys J 70: A141
Reimers JR and Hush NS (1995) Nature of the ground and first excited states of the radical cations of photosynthetic bacterial reaction centers. Chem Phys 197: 323–332
Rigby SEJ, Thapar R, Evans MCW and Heathcote P (1994) The primary donor of Chlorobium limicola f. sp. thiosulphatophilum photosynthetic reaction center. FEBS Lett 350: 24–28
Schubert WD, Klukas O, Krauss N, Saenger W, Fromme P and Witt HT (1995) Present state of the crystal structure analysis of Photosystem I at 4.5 Å resolution. In: Mathis P (ed) Photosynthesis: From Light to Biosphere, Vol II, pp 3–10. Kluwer Academic Publishers, Dordrecht, The Netherlands
Sétif P (1992) Energy transfer and trapping in Photosystem I. In: Barber J (ed) The Photosystems: Structure, Function and Molecular Biology, pp 471–499. Elsevier Science Publishers, Amsterdam
Shimomura ET, Phillippi MA, Goff HM, Scholz WF and Reed CA (1981) Infrared spectroscopy of oxidized metalloporphyrins: Detection of a band diagnostic of porphyrin-centered oxidation. J Am Chem Soc 103: 6778–6780
Sieckmann I, Brettel K, Bock C, van der Est A and Stehlik D (1993) Transient electron paramagnetic resonance of the triplet state of P700 in Photosystem I: Evidence for triplet delocalization at room temperature. Biochemistry 32: 4842–4847
Tavitian BA (1987) Etude par spectroscopie différentielle IRTF des réactions primaires de la photosynthèse. PhD Thesis, Université Pierre et Marie Curie, Paris VI
Tavitian BA, Nabedryk E, Mäntele W and Breton J (1986) Light-induced Fourier transform infrared (FTIR) spectroscopic investigations of primary reactions in Photosystem I and Photosystem II. FEBS Lett 201: 151–157
Thibodeau DL, Nabedryk E and Breton J (1991) Light-induced polarized FTIR spectroscopy of oriented photosynthetic bacterial reaction centers. In: Hester RE and Girling RB (eds) Spectroscopy of Biological Molecules, pp 69–70. The Royal Society of Chemistry, Cambridge
Williams JC, Steiner LA, Feher G and Simon MI (1984) Primary structure of the L subunit of the reaction center from Rhodopseudomonas sphaeroides. Proc Natl Acad Sci USA 81: 7303–7307
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nabedryk, E., Leibl, W. & Breton, J. FTIR spectroscopy of primary donor photooxidation in Photosystem I, Heliobacillus mobilis, and Chlorobium limicola. Comparison with purple bacteria. Photosynth Res 48, 301–308 (1996). https://doi.org/10.1007/BF00041021
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00041021