Abstract
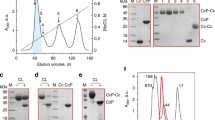
Ferredoxin and the flavoprotein, ferredoxin: NADP reductase, have been covalently linked by incubation in the presence of a water soluble carbodiimide. The cross-linking reaction yields an adduct having a 1:1 stoichiometry. The adduct has depressed levels of diaphorase and NADPH oxidase activity and is inactive in reduction of cytochrome c using NADPH as an electron donor. Thus, although similar to an adduct described by Zanetti and coworkers [J Biol Chem 259: 6153–6157 (1984)] in its stoichiometry, the adduct described herein has significantly different enzymatic properties. It is suggested that this may be a reflection of differences in the interaction between the two proteins resulting from differences in experimental conditions in which the two adducts were prepared.
Similar content being viewed by others
Abbreviations
- Fd:
-
ferredoxin
- Fp:
-
ferredoxin: NADP reductase
- Fd:
-
Fp covalently linked Fd-Fp adduct
- Fd:Fp:
-
noncovalently linked complex between Fd and Fp
- EDC:
-
1-ethyl-3-(dimethylaminopropyl) carbodiimide
- Tris:
-
tris-hydroxymethylaminomethane
- MOPS:
-
3-(N-morpholino)propane sulfonic acid
- DCIP:
-
2,6-dichloropenolindophenol
References
BatieC and KaminH (1981) The relation of pH and oxidation reduction potential to the association state of the ferredoxin-ferredoxin: NADP reductase complex. J Biol Chem 256: 7756–7763
BatieC and KaminH (1984) Ferredoxin: NADP oxidoreductase, equilibria in binary and ternary complexes with NADP and ferredoxin. J Biol Chem 259: 8832–8839
DavisDJ and San PietroA (1977a) Interactions between spinach ferredoxin and other electron carriers. Arch Biochem Biophys 182: 266–272
DavisDJ and San PietroA (1977b) Evidence for the role of sulfhydryl groups in a pH dependent transition of ferredoxin: NADP oxidoreductase. Arch Biochem Biophys 184: 197–203
Davis DJ (1981) Relationship between substrate binding sites on ferredoxin: NADP reductase. Proc 5th Intl Cong Photosynthesis (ed. G Akoyunoglou) 2: 39–46
DavisDJ and HoughK (1983) Preparation of a covalently linked adduct between plastocyanin and cytochrome f. Biochem Biophys Res Commun 116: 1000–1006
Forti G, Cappelletti A, Nobili RL, Garlaschi FM, Gerola PD and Jennings RC 91983) Interaction of ferredoxin and ferredoxin: NADP reductase with thylakoids. Arch Biochem BViophys 221: 507–513
FortiG and BracaleM (1984) Ferredoxin-ferredoxin: NADP reductase interaction, catalytic differences between soluble and thylakoid bound complex. FEBS Lett 166: 81–84
FoustG, MayhewS and MasseyV (1969) Complex formation between ferredoxin: TPN reductase and electron transfer proteins. J Biol Chem 244: 964–970
GerenLM, StonehuernerJ, DavisDJ and MillettFS (1983) The use of a water-soluble carbodiimide to cross-link cytochrome c to plastocyanin. Biochim Biophys Acta 724: 62–68
GerenLM, O'BrienP, StonehuernerJ and MillettFS (1984) identification of specific carboxylate groups on adrenodoxin that are involved in the interaction with adrenodoxin reductase. J Biol Chem 259: 2155–2160
GozzerC, ZanettiG, GallianoM, SacchiGA, MinchiottiL and CurtiB (1977) molecular heterogeneity of ferredoxin: NADP reductase from spinach leaves. Biochim Biophys Acta 485: 278–290
HackettCS and StrittmatterP (1984) Covalent cross-linking of the active sites of vesicle bound cytochrome b5 and NADH: cytochrome b5 reductase. J Biol Chem 259: 3275–3282
MillettFS, Darley-UsmarV and CapaldiR (1982) Cytochrome c is cross-linked to subunit II of cytochrome c oxidase by a water soluble carbodiimide. Biochemistry 21: 3857–3862
NakamuraS (1970) Initiation of sulfite oxidation by ferredoxin: NADP reductase and ferredoxin system: a model experiment on the superoxide anion radical production by metalloflavoproteins. Biochem Biophys Res Commun 41: 177–183.
NakamuraS and KimuraT (1971) Studies on spinach ferredoxin: NADP reductase: Kinetic studies on the interaction of the reductase and ferredoxin and a possible regulation by ionic strength. J Biol Chem 246: 6235–6241
NakamuraS and KimuraM (1972) Studies on aggregated multienzyme systems: Stimulation of Oxygen Uptake of ferredoxin: NADP reductase-ferredoxin complex by cytochrome c. J Biol Chem 247: 6462–6468.
NelsonN and NeumanJ (1969a) Interaction between ferredoxin and ferredoxin: NADP reductase in pyridine nucleotide photoreduction and some partial reactions I. J Biol Chem 244: 1926–1931
NelsonN and NeumanJ (1969b) Interaction between ferredoxin and ferredoxin: NADP reductase in pyridine nucleotide photoreduction and some partial reactions II. J Biol Chem 244: 1932–1936
PeteringDH and PalmerG (1970) Properties of spinach ferredoxin in anaerobic urea solutions: comparison with the native protein. Arch Biochem Biophys 141: 456–464
ShinM (1968) Ferredoxin-NADP reductase. In: YagiK (ed) Flavins and Flavoproteins pp 1–5 Univ Tokyo Press Tokyo Japan
ShinM and SanPietro A (1968) Complex formation of ferredoxin: NADP reductase with ferredoxin and NADP. Biochem Biophys Res Commun 33: 33–42
VieiraBJ and DavisDJ (1983) Chemical modification of spinach ferredoxin with diethylpyrocarbonate: evidence for the essential nature of the histidyl residue. Biochem Biophys Res Commun 112: 508–514
VieiraBJ and DavisDJ (1985) Chemical modification of carboxyl groups on spinach ferredoxin alters its ability to interact with ferredoxin: NADP reductase. Biochem Biophys Res Commun 129: 467–471
VieiraBJ and DavisDJ (1986) Interaction of ferredoxin with ferredoxin: NADP reductase: effects of chemical modification of ferredoxin. Arch Biochem Biophys 247: 140–146
VieiraBJ, ColvertK and DavisDJ (1986) Chemical modification and cross-linking as probes of regions on ferredoxin involved in its interaction with ferredoxin: NADP reductase. Biochim Biohys Acta 851: 109–122
WaldmeyerB, BechtoldR, BosshardHR and PoulosT (1982) The cytochrome c peroxidase-cytochrome c electron transfer complex: experimental evidence for a hypothetical model. J Biol Chem 257: 3073–3076
WeberK and OsbornM (1969) The reliability of molecular weight determinations by dodecyl sulfate polyacrylamide gel electrophoresis. J Biol Chem 244: 4406–4412
ZanettiG, AlivertiA and CurtiB (1984) A cross-linked complex between ferredoxin and ferredoxin: NADP reductase. J Biol Chem 259: 6253–6257
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Colvert, K.K., Davis, D.J. Characterization of a covalently linked complex involving ferredoxin and ferredoxin: NADP reductase. Photosynth Res 17, 231–245 (1988). https://doi.org/10.1007/BF00035450
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00035450