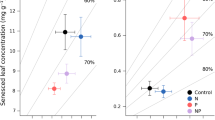
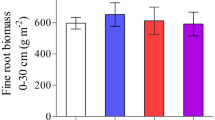
Abstract
Addition of N to an initially N-limited forest increases foliage biomass, demand for water and the probability of water stress. Effects of water and N on tree growth are thus compounded. The 13C abundance of plant tissues is directly correlated with water use efficiency (WUE), and could be used to disentangle the effect of water alone on carbon fixation. However, the 13C abundance may also be directly influenced by changes in rates of photosynthesis related to variations in N status, and by variations in N metabolism via non-RuBisCo carboxylations, and indirectly by effects of N source on WUE. We studied the 13C abundance of current needles from top whorls in two long-term fertilization experiments, one in Norway spruce (Picea abies Karst.) and one in Scots pine (Pinus sylvestris L.). As predicted, N fertilization increased foliage biomass and δ (‰). In the experiment with spruce this effect on 13C abundance was correlated with volume production and foliage biomass in a dry year, but was not seen in a wet year after 19 years of continuous annual N fertilization, which rules out the possible influences of N metabolism and changes in rates of photosynthesis. In the experiment with pine, which was at a drier site, needles from N-fertilized plots had a higher 13C abundance in three dry years, but not significantly so in a wet year. We suggest that effects of N source (NH4 + or NO3 −) on 13C abundance are unlikely to be important under these experimental conditions. The balance between demand and supply of water should thus be the major determinant of the 13C abundance of current needles on top whorls. This opens possibilities to conduct retrospective studies of the role of water supply in fertilization experiments.
Similar content being viewed by others
References
Aber J D, Nadelhoffer K J, Steudler P and Melillo J M 1989 Nitrogen saturation in northern forest ecosystems. BioScience 39, 378–386.
Ågren G I 1983 Nitrogen productivity of some conifers. Can. J. For. Res. 13, 494–500.
Axelsson B 1985 Biomass dynamics in the nutrition experiment at Stråsan. J. Roy. Swed. Acad. Agric. For., Suppl. 17, 30–39.
Barrie A 1991 New methodologies in stable isotope analysis. In Stable Isotopes in Plant Nutrition, Soil Fertility and Environmental Studies. pp 3–25. IAEA, Vienna.
Brix H 1972 Nitrogen fertilization and water effects on photosynthesis and earlywood-latewood production in Douglas-fir. Can. J. For. Res. 2, 467–478.
Ehleringer J R, Field C B, Lin Z and Kuo C Y 1986 Leaf carbon isotope and mineral composition in subtropical plants along an irradiance cline. Oecologia 70, 520–526.
Ericsson A 1978 Seasonal changes in translocation of 14C from different age-classes of needles on 20-year-old Scots pine trees (Pinus sylvestris). Physiol. Plant. 43, 351–358.
Farquhar G D 1991 Use of stable isotopes in evaluating plant water use efficiency. In Stable Isotopes in Plant Nutrition, Soil Fertility and Environmental Studies. pp 475–488. IAEA, Vienna.
Farquhar G D, O'Leary M H and Berry J A 1982 On the relationship between carbon isotope discrimination and photosynthesis. Aust. J. Plant. Physiol. 9, 121–137.
Farquhar G D and Richards P A 1984 Isotopic composition of plant carbon correlates with water use efficiency of wheat genotypes. Aust. J. Plant. Physiol. 11, 339–352.
Field C B and Mooney H A 1986 The photosynthesis-nitrogen relationships in wild plants. In On the Economy of Plant Form and Function. Ed. T Givnish. pp 22–55. Cambridge University Press, Cambridge.
Francey R J, Gifford R M, Sharkey T D and Weir B 1985 Physiological influences on carbon isotope discrimination in huon pine (Lagarostrobos franklinii). Oecologia 66, 211–218.
Garten C T and Taylor G E Jr 1992 Foliar δ13C within a temperate deciduous forest: Spatial, temporal and species sources of variation. Oecologia 90, 1–7.
Högberg P, Johannisson C, Nicklasson H and Högbom L 1990 Shoot nitrate reductase activities of field-layer species in different forest types. I. Scand. J. For. Res. 5, 449–456.
Högberg P, Tamm C-O and Högberg M 1992 Variations in 15N abundance in a forest fertilization trial: Critical loads of N, N saturation, contamination and effects of revitalization fertilization. Plant and Soil 142, 211–219.
Linder S, Benson M L, Myers B J and Raison R J 1987 Canopy dynamics and growth of Pinus radiata. I. Effects of irrigation and fertilization during a drought. Can. J. For. Res. 17, 1157–1165.
Lindroth A 1987 Kvävetillförsel kan orsaka kronutglesning. Sveriges Skogsvårdsförbunds Tidsskrift 3, 9–17.
Medina E and Minchin P 1980 Stratification of δ13C values of leaves in Amazonian rain forest. Oecologia 45, 377–378.
Raven J A and Farquhar G D 1990 The influence of N metabolism and organic acid synthesis on the natural abundance of isotopes of carbon in plants. New Phytol. 116, 505–529.
Raven J A, Wollenweber B and Handley L L 1992 A comparison of ammonium and nitrate as nitrogen sources for photolithotrophs. New Phytol. 121, 19–32.
Read D J 1983 The biology of mycorrhiza in the Ericales. Can. J. Bot. 61, 985–1004.
Tamm C-O 1964 Determination of nutrient requirements of forest stands. Int. Rev. For. Res. 1, 115–170.
Tamm C-O 1985 The Swedish optimum nutrition experiments in forest stands-aim, methods, yield, results. J. Roy. Swed. Acad. Agric. For., Suppl. 17, 9–29.
Tamm C-O 1991 Nitrogen in Terrestrial Ecosystems. Ecological Studies No. 81. Springer-Verlag, Berlin. 115 p.
Tamm C-O, Aronsson A and Burgtorf H 1974a The optimum nutrition experiment Stråsan: A brief description of an experiment in a young stand of Norway spruce (Picea abies Karst.). Res. Note No. 17. Dep. For. Ecol. For. Soils, Sch. For., Stockholm, Sweden. 29 p.
Tamm C-O, Nilsson Å and Wiklander G 1974b The optimum nutrition experiment Lisselbo: A brief description of an experiment in a young stand of Scots pine (Pinus sylvestris L.). Res. Note No. 18. Dep. For. Ecol. For. Soils, Sch. For., Stockholm, Sweden. 25 p.
Vogel J C 1978 Recycling of carbon in a forest environment. Oecol. Plant. 13, 89–94.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Högberg, P., Johannisson, C. & Hällgren, JE. Studies of 13C in the foliage reveal interactions between nutrients and water in forest fertilization experiments. Plant Soil 152, 207–214 (1993). https://doi.org/10.1007/BF00029090
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00029090