Summary
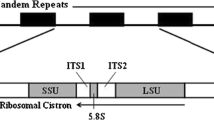
We have characterized the nuclear rDNA unit of Euglena gracilis var. bacillaris and compared it to that of the Z strain. We have localized restriction sites for Eco R1, Sal 1, Sma 1, Hind III, Bam H1 and Bgl II on this unit as well as the coding region for 20 S and 25 S rRNAs. For both strains, results suggest an homogeneity of the 11.6 kbp rDNA units. Comparison between strains shows differences characterized by two additional Sal 1 sites in bacillaris and the likely methylation of one Sma 1 site in Z. Both differences are localized in a non-coding region of the rDNA unit. Analyses of 18 Euglena strains from various origins confirm these differences and allow easy recognition of bacillaris and Z type strains.
Similar content being viewed by others
Abbreviations
- kb:
-
kilo base
- kpb:
-
kilo base pair
- plasmids:
-
pRH 59 and pRH 57 contain a Hind III-HInd III nuclear DNA fragment from W3BUL of 5.9 and 5.7 kbp respectively, pRB 48 and pRB 35 contain a Bam H1-Bam H1 nuclear DNA fragment from wild-type Z of 4.8 and 3.5 kbp respectively
- SDS:
-
sodium dodecyl sulfate
- UV:
-
ultra-violet
References
Birnboim HC, Doly J: A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res 7:1513–1523, 1979.
Brosius J, Palmer ML, Kennedy PJ, Noller HF: Complete nucleotide sequence of a 16 S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci USA 75:4801–4805, 1978.
Brosius J, Dull TJ, Noller HF: Complete nucleotide sequence of a 23 S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci USA 77:201–204, 1980.
Curtis SE, Rawson JRY: Characterization of the nuclear ribosomal DNA of Euglena gracilis. Gene 15:237–247, 1981.
Davis RW, Simon M, Davidson N: Electron microscope heteroduplex methods for mapping regions of base sequence homology in nucleic acids. Methods in Enzymology 21:413–428, 1971.
Flamant E, Heizmann P, Nigon V: Rearrangement of chloroplast ribosomal cistrons by unequal crossing-over in Euglena gracilis. Curr Genet 8:9–13, 1984.
Freyssinet G, Freyssinet M, Lebrun M: Occurrence of the gene for the large subunit of ribulose-1,5-bisphosphate carboxylase in several mutants of Euglena gracilis. Plant Sci Lett 37:247–249, 1985.
Freyssinet G, Neyret O, Leburn M. Freyssinet M, Rouly JC: A simple, inexpensive apparatus for electroelution of nucleic acids of proteins from agarose or polyacrylamide gels. Physiol Vég 22:897–904, 1984.
Graf L, Roux E, Stutz E: Nucleotide sequence of Euglena gracilis chloroplast gene coding for the 16 S rRNA: homologies to E. coli and Zea mays chloroplast 16 S rRNA. Nucleic Acids Res 10:6369–6381, 1982.
Greenbaltt CL, Schiff JA: A pheophytin like pigment in dark-adapted Euglena gracilis. J Protozool 6:23–28, 1959.
Grunstein M, Hogness DS: Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci USA 72:3961–3965, 1975.
Hallick RB: Chloroplast DNA. In: Buetow DE (ed) The Biology of Euglena, Vol 4. Academic Press. In press.
Helling RB, El-Gewely MR, Lomax MI, Baumgartner JE, Schwartzbach SD, Barnett WE: Organization of the chloroplast ribosomal RNA genes of Euglena gracilis bacillaris. Molec Gen Genet 174:1–10, 1979.
Heizmann P, Doly J, Hussein Y, Nicolas P, Nigon V, Bernardi G: The chloroplast genome of bleached mutants of Euglena gracilis. Biochim Biophys Acta 653:412–415, 1981.
Koller B, Delius H: Chloroplast DNA of Euglena gracilis with five complete rRNA operons and two extra 16 S rRNA genes. Molec Gen Genet 188:305–308, 1982.
Koller B, Delius H, Helling RB: Structure and rearrangements of rRNA genes in chloroplast DNA in two strains of Euglena gracilis. Plant Mol Biol 3:127–136, 1984.
Meeker RR, Thomas JR, Tewari KK: In vitro iodination of plant ribonucleic acids. Plant Physiol 58:71–76, 1976.
Nicolas P, Heizmann P, Ravel-Chapuis P, Flamant F, Nigon V: Le génome chloroplastique des cellules sauvages et des mutants non-photosynthétiques d'Euglena gracilis. Ann Biol 23:97–145, 1984.
Perler F, Efstratiadis A, Lomedico P, Gilbert W, Kolodner R, Dodgson J: The evolution of genes: the chicken preproinsulin gene. Cell 20:555–566, 1980.
Ravel-Chapuis P, Flamant F, Nicolas P, Heizmann P, Nigon V: Diversity of the ribosomal structures in the Euglena gracilis chloroplast genome: description of a mutant with two ribosomal operons and possible mechanisms for its production. Nucleic Acids Res 12:1039–1048, 1984.
Rawson JRY, Crouse EJ, Stutz E: The integrity of the 25 S ribosomal RNA from Euglena gracilis 87 S ribosomes. Biochim Biophys Acta 246:507–516, 1971.
Russell GK, Draffan AG, Schmidt GW, Lyman H: Light-induced formation in a chlorophyll-less mutant of Euglena gracilis. Plant Physiol 62:678–682, 1978.
Salvador G, Richard F, Nicolas P, Nigon V: Structures et propriétés d'un nouveau mutant blanc d'Euglena gracilis. Protistologica 8:533–540, 1972.
Schiff JA, Lyman H, Russell GK: Isolation of mutants from Euglena gracilis. Methods in Enzymology 23:143–162, 1971.
Schiff JA, Lyman H, Russell GK: Isolation of mutants from Euglena gracilis. Methods in Enzymology 69:23–29, 1980.
Scott NS, Kavanagh TA, Timmis JN: Methylation of rRNA genes in some higher plants. Plant Science Lett 35:213–217, 1984.
Smith GE, Summers MD: The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem 109:123–129, 1980.
Uchimiya H, Kato H, Ohgawara T, Harada H, Sugiura M: Sequence-specific methylation of ribosomal RNA genes contained in the nuclear DNA of tobacco. Plant Cell Physiol 23:1129–1131, 1982.
Verdier G, Trabuchet G, Heizmann P, Nigon V: Effet de l'éclairement sur les synthèses de RNA et de séquences polyadényliques dans des cultures d'Euglena gracilis étiolées. Biochim Biophys Acta 312:528–539, 1973.
Wellauer PK, Dawid IB: The structural organization of ribosomal DNA in Drosophila melanogaster. Cell 10:193–212, 1977.
Wurtz EA, Buetow DE: Intraspecific variation in the structural organization and redundancy of chloroplast ribosomal DNA cistrons in Euglena gracilis. Current Genet 3:181–187, 1981.
Zurawski G, Perrot B, Bottomley W, Whitfeld PR: The structure of the gene for the large subunit of ribulose-1,5-bisphosphate carboxylase from spinach chloroplast DNA. Nucleic Acids Res 8:3251–3270, 1981.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Neyret-Djossou, O., Freyssinet, G., Ravel-Chapuis, P. et al. Comparison between the organization of nuclear ribosomal DNA unit of Euglena gracilis Z and var. Bacillaris . Plant Mol Biol 6, 111–117 (1986). https://doi.org/10.1007/BF00027304
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00027304