Abstract
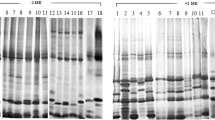
This study concerned the molecular basis for the protein size heterogeneity of extensin from two maize (Zea mays L.) varieties. We studied the physical properties of extensin, a hydroxyproline-rich glycoprotein (HRGP), from the silk and pericarp of Golden X Bantam (GXB) sweet corn and Japanese Hulless (JHL) popcorn. Extensin from GXB has a molecular mass of 66 kDa whereas extensins from JHL have molecular masses of 76 and 66 kDa. Treatment with anhydrous hydrogen fluoride to deglycosylate proteins reduced the size of all extensins by 5 kDa. Probing with a 500 bp fragment from a genomic clone of maize extensin identified two transcripts (1.9 and 1.5 kb) on northern blots. JHL contained both transcripts and GXB contained only the 1.5 kb transcript. The probe also hybridized to two larger transcripts (6.2 and 4.5 kb) that were found in both varieties. We immunoprecipitated two proteins (66 and 56 kDa) from translated RNA isolated from JHL and one protein (56 kDa) from GXB. These results demonstrate that these extensins differ in the size of their peptide moiety and not in their extent of glycosylation.
Similar content being viewed by others
References
Budowle B, Deadman HA, Murch RS, Baechtel FS: An introduction to the methods of DNA analysis under investigation in the FBI laboratory. Crime Lab Digest 15: 3–211 (1988).
Fritz SE, Hood KR, Hood EE: Localization of soluble and insoluble fractions of hydroxyproline-rich glycoproteins during maize kernel development. J Cell Sci 98: 545–550 (1991).
Haffner MH, Chin MB, Lane BG: Wheat embryo ribonucleates. XII. Formal characterization of terminal and penultimate nucleoside residues at the 5′-ends of ‘capped’ RNA from imbibing wheat embryos. Can J Biochem 56: 729–733 (1978).
Hood EE, Shen QX, Varner JE: A developmentally regulated hydroxyproline-rich glycoprotein in maize pericarp cell walls. Plant Physiol 87: 138–142 (1988).
Hood EE, Hood KR, Fritz SE: Hydroxyproline-rich glycoproteins in cell walls of pericarp from maize. Plant Sci 79: 13–22 (1991).
Hood KR, Baasiri RA, Fritz SE, Hood EE: Biochemical and tissue print analyses of hydroxyproline-rich glycoproteins in cell walls of sporophytic maize tissues. Plant Physiol 96: 1214–1219 (1991).
Kieliszewski MJ, Lamport DTA: Purification and partial characterization of a hydroxyproline-rich glycoprotein in graminaceous monocot, Zea mays. Plant Physiol 85: 823–827 (1987).
Kieliszewski MJ, Leykam JF, Lamport DTA: Structure of the threonine-rich extensin from Zea mays. Plant Physiol 92: 316–326 (1990).
Laemmli UK: Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227: 680–685 (1970).
Lamport DTA: In: Loewus FA, Runeckles BC (eds) Recent Advances in Phytochemistry, Volume 2, pp. 79–115. Plenum, New York (1977).
Laskey RA, Mills AD: Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem 56: 335–341 (1975).
Maniatis T, Fritsch EF, Sambrook J: Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY (1982).
Matsudaira P: Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem 262: 10035–10038 (1987).
Mort AJ, Lamport DTA: Anhydrous hydrogen fluoride deglycosylates glycoproteins. Anal Biochem 82: 289–309 (1977).
Nielson BL, Brown LR: The basis for colored silver-protein complex formation in stained polyacrylamide gels. Anal Biochem 141: 311–315 (1984).
Rossier C, Franke J, Mullens IA, Kelley KJ, Kessen RH: Detection and regulation of the mRNA for the inhibitor of extracellular cAMP phosphodiesterase of Dictyostelium discoideum. Eur J Biochem 133: 383–391 (1983).
Ruiz-Avila L, Ludevid MD, Puigdomenech P: Differential expression of a hydroxyproline-rich cell-wall protein gene in embryonic tissues of Zea mays L. Planta 184: 130–136 (1991).
Showalter AM, Varner JE: Molecular details of plant cell hydroxyproline-rich glycoprotein expression during wounding and infection. In: Molecular Strategy for Crop Protection, pp. 375–392. Alan R. Liss, New York (1987).
Showalter AM, Varner JE: Plant hydroxyproline-rich glycoproteins. In: Stumpf PK, Conn EE (eds) Biochemistry of Plants: A Comprehensive Treatise, Volume 15, pp. 485–520. Academic Press, New York (1989).
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC: Measurement of protein using bicinchoninic acid. Anal Biochem 150: 76–85 (1985).
Stiefel V, Perez-Grau L, Alberico F, Giralt E, Ruiz-Avila L, Ludevid MD, Puigdomenech P: Molecular cloning of cDNAs encoding a putative cell wall protein from Zea mays and immunological identification of related polypeptides. Plant Mol Biol 11: 483–493 (1988).
Stiefel V, Ruiz-Avila L, Raz R, Vailes MP, Gomez J, Pages M, Martinez-Izquierdo JA, Ludevid MD, Langdale JA, Nelson T, Puigdomenech P: Expression of a maize cell wall hydroxyproline-rich glycoprotein gene in early leaf and root vascular differentiation. Plant Cell 2: 785–793 (1990).
Thomas JM, Hodes ME: A new discontinous buffer system for electrophoresis of cationic proteins at nearneutral pH. Anal Biochem 118: 194–196 (1981).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Murphy, J.M., Hood, E.E. Molecular basis for extensin size heterogeneity in two maize varieties. Plant Mol Biol 21, 885–893 (1993). https://doi.org/10.1007/BF00027119
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00027119