Abstract
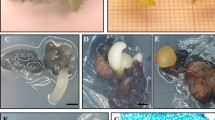
Somatic embryos were obtained from leaf discs of juvenile red oak plants. Basal inductive nutrient medium was a modified Murashige and Skoog solution enriched with 500 mg L−1 casein hydrolysate, 100 mg L−1 polyvinylpyrrolidone, 5.4 μM naphthaleneacetic acid and 0.09 μM benzyladenine. Embryogenesis was obtained only from leaf discs in the presence of light and increased when the adaxial surface of the explants (with midrib or main veins present) was in contact with the medium. Large variation was observed in all experiments. Recurrent embryogenesis was observed at the base of embryo clusters with callus present; conversely, embryogenic potential was rapidly lost by subculturing full calli. Maturation, germination and development of isolated somatic embryos were obtained. However, the vast majority of embryos did not have viable apical bud meristems and on only a few occasions were shoots produced.
Similar content being viewed by others
Abbreviations
- BA:
-
N6-benzyladenine
- CH:
-
casein hydrolysate
- 2iP:
-
isopentenyladenine
- NAA:
-
naphthaleneacetic acid
- 2.4-D:
-
2.4-dichlorophenoxyacetic acid
- GA:
-
gibberellic acid
- PVP:
-
polyvinylpyrrolidone
References
Bennett L (1986) Tissue culture of oaks and redbuds. Proc Int Plant Prop Soc 36: 421–426
Bonga JM (1982) Vegetative propagation in relation to juvenility, maturity and rejuvenation. In: Bonga JM and Durzan DJ (eds) Tissue Culture in Forestry, pp 387–412. Martinus Nijhoff/Dr W. Junk Publishers, The Hague, the Netherlands
Brisson M, David A, Lamant A and Rancillac M (1994) Les essais de multiplication in vitro. In: Timbal J, Kremer A, LeGoff N and Nepveu G (eds) Le chêne rouge d'Amérique, pp 460–468, 515–516. INRA-Editions, Versailles, France
Bueno M, Astorga R and Manzanera J (1992) Plant regeneration through somatic embryogenesis in Quercus suber. Physiol Plant 85: 30–34
Chalupa V (1993) Vegetative propagation of oak (Quercus robur and Q. petraea) by cutting and tissue culture. Ann Sci For 50, Suppl 1: 295S-307S
El Maâtaoui M, Espagnac H and Michaux-Ferrière N (1990) Histology of callogenesis and somatic embryogenesis induced in stem fragments of cork oak (Quercus suber) cultured in vitro. Ann Bot 66: 183–190
Féraud-Keller C and Espagnac H (1989) Conditions d'apparition d'une embryogenése somatique sur des cals issus de la culture de tissus foliaires du chêne vert (Quercus ilex) Can J Bot 67: 1066–1070
Gingas VM and Lineberger RD (1989) Asexual embryogenesis and plant regeneration in Quercus. Plant Cell Tissue Organ Cult 17: 191–203
Gingas VM (1991) Asexual embryogenesis and plant regeneration from male catkins of Quercus. Hort Sci 26: 1217–1218
Heller R (1953) Recherches sur la nutrition minérale des tissus végétaux in vitro. Ann Sci Nat Bot Biol Vég 14: 1–223
Jörgensen J (1993) Embryogenesis in Quercus petraea. Ann Sci For 50, Suppl 1: 344S-350S
Label P and Lelu M-A (1994) Influence of exogenous abscisic acid on germination and plantlet conversion frequencies of hybrid larch somatic embryos (Larix × leptoeuropaea). Plant Growth Regul 15: 175–182
Manzanera JA and Pardos JA (1990) Micropropagation of juvenile and adult Quercus suber L. Plant Cell Tissue Organ Cult 21: 1–8
Morel G and Martin C (1955) Guérison de pommes de terre atteintes de maladie à virus. C R Acad Agric 41: 472–475
Murashige T and Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497
Rancillac M, Klinguer A and Klinguer S (1991) Plant biotechnologies applied to a forest tree, the American red oak (Quercus rubra L.) Acta Hort 289: 341–342
Seckinger GR, McCown BH and Struckmeyer BE (1979) Production of anomalous structures in Quercus rubra L. callus cultures. Am J Bot 66: 993–996
Srivastava PS and Steinhauer A (1982) In vitro culture of embryo segments of Quercus lebani: organogenesis and callus growth as a differential response to experimental conditions. Z Pflanzenphysiol 106: 93–96
Szczotka Z (1984) Polyamines changes in (Quercus borealis Michx) and (Quercus robur L) seeds during ageing in controlled conditions. Acta Physiol Plant 6: 127–135
Timbal J, Kremer A, LeGoff N and Nepveu G (1994) Le 083 chêne rouge d'Amérique, pp 1–564. INRA-Editions, Versailles, France
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rancillac, M., Klinguer, A., Klinguer, S. et al. Preliminary investigations on somatic embryogenesis from leaf discs of red oak (Quercus rubra L.). Plant Growth Regul 20, 67–73 (1996). https://doi.org/10.1007/BF00024061
Issue Date:
DOI: https://doi.org/10.1007/BF00024061