Abstract
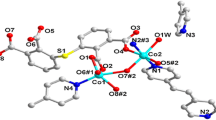
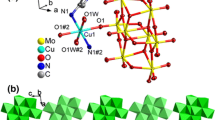
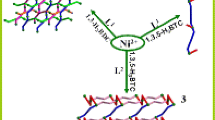
Coordination to form polymer is emerging as a new technology for modifying or enhancing the properties of the existed energetic substances in energetic materials area. In this work, guanidine cation CN3H6+ (Gu) and 3-amino-1,2,4- triazole C2H4N4 (ATz) were crystallized into NaN5 and two novel energetic coordination polymers (CPs), (NaN5)5[(CH6- N3)N5](N5)3– (1) and (NaN5)2(C2H4N4) (2) were prepared respectively via a self-assembly process. The crystal structure reveals the co-existence of the chelating pentazole anion and organic component in the solid state. In polymer 1, Na+ and N5– were coordinated to form a cage structure in which guanidine cation [C(NH2)3]+ was trapped; for polymer 2, a mixedligand system was observed; N5– and ATz coordinate separately with Na+ and form two independent but interweaved nets. In this way, coordination polymer has been successfully utilized to modify specific properties of energetic materials through crystallization. Benefiting from the coordination and weak interactions, the decomposition temperatures of both polymers increase from 111°C (1D structure [Na(H2O)(N5)] ∙2H2O) to 118.4 and 126.5°C respectively. Moreover, no crystallized H2O was generated in products to afford the anhydrous compounds of pentazole salts with high heats of formation (>800 kJ mol–1). Compared to traditional energetic materials, the advantage in heats of formation is still obvious for the cyclo-N5– based CPs, which highlights cyclo-N5– as a promising energetic precursor for high energy density materials (HEDMs).
摘要
在含能材料领域, 通过协同作用形成聚合物已成为改善或增强现有含能物质性能的一种新技术. 本文将胍阳离子CH3H6+(GU)和氨基-1,2,4-三唑C2H4N4(ATZ)与NaN5一起结晶, 通过自组装过程分别制备了两种新型含能配位聚合物(CPs), (NaN5)5[(CH3H6)N5](N5)3 (1)和(NaN5)2(C2H4N4) (2). 晶体结构表明, 在固体状态下, 螯合的五唑阴离子实现了与其他有机成分共存. 聚合物1, Na+和N5−形成笼状, 并将胍阳离子[C(NH2)3]+围在里面; 而聚合物2是一个混合配体体系, N5−和ATZ与Na+分别形成两个独立但相互交织的网. 这些都说明了通过结晶形成配位聚合物, 来改变含能材料的特定性能是可行的. 受益于配位和弱相互作用, 两种聚合物的热分解温度分别从111°C (一维结构[Na(H2O)(N5)]·2H2O)提高到了118.4和126.5°C. 此外, 他们成功地除去了产物中的结晶水, 成为具有高生成热特点的五唑无水盐(> 800 kJ mol−1). 聚合物1和2比传统能量材料高得多的生成热, 表明N5−作为高能量密度材料(HEDMs)的前驱体, 具有很好的前景.
Similar content being viewed by others
References
Christe KO. Polynitrogen chemistry enters the ring. Science, 2017, 355: 351–351
Klapötke TM. Chemistry of High-Energy Materials. Boston: De Gruyter, 2017
Yin P, Shreeve JM. Advances in heterocyclic chemistry. London: Elsevier, 2017, 121: 89–131
Zhang Q, Shreeve J’M. Energetic ionic liquids as explosives and propellant fuels: a new journey of ionic liquid chemistry. Chem Rev, 2014, 114: 10527–10574
Singh RP, Gao H, Meshri DT, Shreeve JM. High Energy Density Materials. Berlin: Springer, 2007, 35–83
Chen SL, Yang ZR, Wang BJ, et al. Molecular perovskite highenergetic materials. Sci China Mater, 2018, doi: 10.1007/s40843-017-9219-9
Gao H, Shreeve J’M. Azole-based energetic salts. Chem Rev, 2011, 111: 7377–7436
Fischer N, Fischer D, Klapötke TM, et al. Pushing the limits of energetic materials—the synthesis and characterization of dihydroxylammonium 5,5’-bistetrazole-1,1’-diolate. J Mater Chem, 2012, 22: 20418–20422
Zhang S, Yang Q, Liu X, et al. High-energy metal–organic frameworks (HE-MOFs): Synthesis, structure and energetic performance. Coord Chem Rev, 2016, 307: 292–312
Li S, Wang Y, Qi C, et al. 3D energetic metal-organic frameworks: synthesis and properties of high energy materials. Angew Chem Int Ed, 2013, 52: 14031–14035
McDonald KA, Seth S, Matzger AJ. Coordination polymers with high energy density: an emerging class of explosives. Cryst Growth Des, 2015, 15: 5963–5972
Zhang J, Shreeve JM. Time for pairing: cocrystals as advanced energetic materials. CrystEngComm, 2016, 18: 6124–6133
Landenberger KB, Bolton O, Matzger AJ. Energetic–energetic cocrystals of diacetone diperoxide (DADP): dramatic and divergent sensitivity modifications via cocrystallization. J Am Chem Soc, 2015, 137: 5074–5079
Haiges R, Boatz JA, Vij A, et al. Polyazide chemistry: preparation and characterization of Te(N3)4 and [P(C6H5)4]2[Te(N3)6] and evidence for [N(CH3)4][Te(N3)5]. Angew Chem Int Ed, 2003, 42: 5847–5851
Fehlhammer WP, Beck W. Azide chemistry—an inorganic perspective, part I metal-azides: overview, general trends and recent developments. Z Anorg Allg Chem, 2013, 639: 1053–1082
Christe KO, Wilson WW, Sheehy JA, et al. N5 +: a novel homoleptic polynitrogen ion as a high energy density material. Angew Chem Int Ed, 1999, 38: 2004–2009
Haiges R, Schneider S, Schroer T, et al. High-energy-density materials: synthesis and characterization of N5 +[P(N3)6]-, N5 +[B(N3)4]-, N5 +[HF2]-·nHF, N5 +[BF4]-, N5 +[PF6]-, and N5 +[SO3F]-. Angew Chem Int Ed, 2004, 43: 4919–4924
Vij A, Pavlovich JG, Wilson WW, et al. Experimental detection of the pentaazacyclopentadienide (pentazolate) anion, cyclo-N5 -. Angew Chem Int Ed, 2002, 41: 3051–3054
Östmark H, Wallin S, Brinck T, et al. Detection of pentazolate anion (cyclo-N5 -) from laser ionization and decomposition of solid p-dimethylaminophenylpentazole. Chem Phys Lett, 2003, 379: 539–546
Steele BA, Oleynik II. Sodium pentazolate: A nitrogen rich high energy density material. Chem Phys Lett, 2016, 643: 21–26
Bazanov B, Geiger U, Carmieli R, et al. Detection of cyclo-N5 -in THF solution. Angew Chem Int Ed, 2016, 55: 13233–13235
Steele BA, Stavrou E, Crowhurst JC, et al. High-pressure synthesis of a pentazolate salt. Chem Mater, 2017, 29: 735–741
Zhang C, Sun C, Hu B, et al. Synthesis and characterization of the pentazolate anioncyclo–N5 -in (N5)6(H3O)3(NH4)4Cl. Science, 2017, 355: 374–376
Xu Y, Wang Q, Shen C, et al. A series of energetic metal pentazolate hydrates. Nature, 2017, 549: 78–81
Zhang C, Yang C, Hu B, et al. A symmetric Co(N5)2(H2O)4·4H2O high-nitrogen compound formed by cobalt(II) cation trapping of a cyclo-N5 -anion. Angew Chem Int Ed, 2017, 56: 4512–4514
Xu Y, Wang P, Lin Q, et al. A carbon-free inorganic–metal complex consisting of an all-nitrogen pentazole anion, a Zn(ii) cation and H2O. Dalton Trans, 2017, 46: 14088–14093
Zhang W, Wang K, Li J, et al. Stabilization of the pentazolate anion in a zeolitic architecture with Na20N60 and Na24N60 nanocages. Angew Chem Int Ed, 2018, 57: 2592–2595
Frisch MJ, Trucks GW, Schlegel HB, et al. Gaussian 09, Revision A.02, Gaussian, Inc, Wallingford CT, 2009
Becke AD. Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys, 1993, 98: 5648–5652
Lee C, Yang W, Parr RG. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B, 1988, 37: 785–789
Lu T, Chen F. Multiwfn: A multifunctional wavefunction analyzer. J Comput Chem, 2012, 33: 580–592
Johnson ER, Keinan S, Mori-Sanchez P, et al. Revealing noncovalent interactions. J Am Chem Soc, 2010, 132: 6498–6506
SAINT v7.68A Bruker AXS Inc: Madison, WI, 2009
Sheldrick GM. SHELXL-2014/7, University of Göttingen, Germany, 2014
SADABS v2008/1 Bruker AXS Inc.: Madison, WI, 2008
Spek AL. PLATON, An integrated tool for the analysis of the results of a single crystal structure determination. Acta Crystallogr sect A, 1990, 46: C34
Kamlet MJ, Jacobs SJ. Chemistry of detonations. I. A simple method for calculating detonation properties of C–H–N–O explosives. J Chem Phys, 1968, 48: 23–35
Zhang Y, Zhang S, Sun L, et al. A solvent-free dense energetic metal–organic framework (EMOF): to improve stability and energetic performance via in situ microcalorimetry. Chem Commun, 2017, 53: 3034–3037
Xu Y, Liu W, Li D, et al. In situ synthesized 3D metal–organic frameworks (MOFs) constructed from transition metal cations and tetrazole derivatives: a family of insensitive energetic materials. Dalton Trans, 2017, 46: 11046–11052
Wang Y, Zhang J, Su H, et al. A simple method for the prediction of the detonation performances of metal-containing explosives. J Phys Chem A, 2014, 118: 4575–4581
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (11702141, 21771108, and U1530101). The authors gratefully acknowledge Dongxue Li (College of Chemical Engineering, Nanjing Tech University) for her tests of the Raman spectra.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Pengcheng Wang obtained his BSc in 2008 and PhD in 2013 at Nanjing University of Science and Technology (NJUST), and was a postdoc at the National Institute of Advanced Industrial Science and Technology (AIST) from 2013 to 2014. He joined NJUST in 2014. His current research interest is in the synthesis and crystal engineering of energetic materials.
Yuangang Xu was born in 1990. He is a PhD candidate of applied chemistry in NJUST. His research focuses on the synthesis of nitrogen-rich energetic materials.
Qiuhan Lin obtained his BSc in 2008 and PhD in 2013 at Beijing Institute of Technology. He joined NJUST as an associate professor in 2014. His current research interest is in the synthesis and crystal engineering of energetic salts.
Ming Lu obtained his BSc in 1984, MSc in 1989 and PhD in 1999 at NJUST. He then became a professor in 2001. His current research interest focuses on the synthesis and crystal engineering of energetic materials, pharmaceutical intermediates and green chemistry.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wang, PC., Xu, YG., Wang, Q. et al. Self-assembled energetic coordination polymers based on multidentate pentazole cyclo-N5−. Sci. China Mater. 62, 122–129 (2019). https://doi.org/10.1007/s40843-018-9268-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-018-9268-0