Abstract
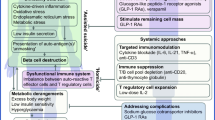
In spite of modern techniques, the burden for patients with type 1 diabetes mellitus will not disappear, and type 1 diabetes will remain a life-threatening disease causing severe complications and increased mortality. We have to learn of ways to stop the destructive process, preserve residual insulin secretion or even improve the disease via β-cell regeneration. This will give a milder disease, a more stable metabolism, simpler treatment and perhaps even cure. Therapies based on single drugs have not shown sufficient efficacy; however, there are several treatments with encouraging efficacy and no apparent, or rather mild, adverse events. As the disease process is heterogeneous, treatments have to be chosen to fit relevant subgroups of patients, and step by step efficacy can possibly be improved by the use of combination therapies. Thus immunosuppressive therapies like anti-CD3 and anti-CD20 monoclonal antibodies might be combined with fusion proteins such as etanercept [tumor necrosis factor (TNF)-α inhibitor] and/or abatacept (CTLA4-Ig) early after onset to stop the destructive process, supported by β-cell protective agents. The effect may be prolonged by using autoantigen therapy [glutamate decarboxylase (GAD) proinsulin], and by adding agents facilitating β-cell regeneration [e.g. glucagon-like peptide-1 (GLP-1)] there should be a good chance to make the disease milder, perhaps leading to cure in some patients.
Similar content being viewed by others
References
The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86.
Bojestig M, Arnqvist HJ, Hermansson G, Karlberg BE, Ludvigsson J. Declining incidence of nephropathy in insulin-dependent diabetes mellitus. N Engl J Med. 1994;330:15–8.
Lind M, Svensson AM, Rosengren A. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med. 2015;372(9):880–1.
Lutterman JA, Benraad TJ, van ‘t Laar A. The relationship between residual insulin secretion and metabolic stability in type 1 (insulin dependent) diabetes. Diabetologia. 1981;21(2):99–102.
Kohnert KD, Augstein P, Zander E, Heinke P, Peterson K, Freyse EJ, Hovorka R, Salzsieder E. Glycemic variability correlates strongly with postprandial beta-cell dysfunction in a segment of type 2 diabetic patients using oral hypoglycemic agents. Diabetes Care. 2009;32(6):1058–62.
Madsbad S, Alberti KG, Binder C, Burrin JM, Faber OK, Krarup T, Regeur L. Role of residual insulin secretion in protecting against ketoacidosis in insulin-dependent diabetes. Br Med J. 1979;2:1257–9.
Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the Diabetes Control and Complications Trial. Diabetes Care. 2003;26:832–6.
Keenan HA, Sun JK, Levine J, Doria A, Aiello LP, Eisenbarth G, Bonner-Weir S, King GL. Residual insulin production and pancreatic ß-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes. 2010;59(11):2846–53.
Wahren J, Ekberg K, Jörnvall H. C-peptide is a bioactive peptide. Diabetologia. 2007;50(3):503–9.
Ludvigsson J. C-peptide an adequate end-point in type 1 diabetes. Diabetes Metab Res Rev. 2009;25(8):691–3.
Rowe PA, Campbell-Thompson ML, Schatz DA, Atkinson MA. The pancreas in human type 1 diabetes. Semin Immunopathol. 2011;33:29–43 (pmid: 20495921).
Pugliese A, Vendrame F, Reijonen H, Atkinson MA, Campbell-Thompson M, Burke GW. New insight on human type 1 diabetes biology: nPOD and nPOD-transplantation. Curr Diab Rep. 2014;14(10):530.
Gepts W, Lecompte PM. The pancreatic islets in diabetes. Am J Med. 1981;70(1):105–15.
Richardson SJ, Morgan NG, Foulis AK. Pancreatic pathology in type 1 diabetes mellitus. Endocr Pathol. 2014;25(1):80–92.
Rodriguez-Calvo T, Ekwall O, Amirian N, Zapardiel-Gonzalo J, von Herrath MG. Increased immune cell infiltration of the exocrine pancreas: a possible contribution to the pathogenesis of type 1 diabetes. Diabetes. 2014;63(11):3880–90.
Ludvigsson J. Is it time to challenge the established theories surrounding type 1 diabetes? Acta Paediatr. 2014;103(2):120–3.
Roep BO, Peakman M. Diabetogenic T lymphocytes in human Type 1 diabetes. Curr Opin Immunol. 2011;23(6):746–53.
Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3 + regulatory T cells. Nat Rev Immunol. 2011;11:119.
Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7.
Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat Rev Endocrinol. 2009;5(4):219–26.
Eizirik DL, Grieco FA. On the immense variety and complexity of circumstances conditioning pancreatic β-cell apoptosis in type 1 diabetes. Diabetes. 2012;61(7):1661–3.
Ludvigsson J, Heding L. Abnormal proinsulin/C-peptide ratio in juvenile diabetes. Acta Diabetol Lat. 1982;19(4):351–8.
Leahy JL, Bonner-Weir S, Weir GC. Beta-cell dysfunction induced by chronic hyperglycemia. Current ideas on mechanism of impaired glucose-induced insulin secretion. Diabetes Care. 1992;15(3):442–55.
Jackson RL, Boyd JD, Smith TE. Stabilization of the diabetic child. Am J Dis Child. 1940;59(2):332–41.
Brush JM. Initial stabilizatin of the diabetic child. Am J Dis Child. 1944;1944(67):429–34.
Ludvigsson J, Heding LG, Larsson Y, Leander E. C-peptide in juvenile diabetics beyond the postinitial remission period. Relation to clinical manifestations at onset of diabetes, remission and diabetic control. Acta Paediatr Scand. 1977;66(2):177–84.
Mirouze J, Selam JL, Pham TC, Mendoza E, Orsetti A. Sustained insulin-induced remissions of juvenile diabetes by means of an external artificial pancreas. Diabetologia. 1978;14(4):223–7.
Diabetes Prevention Trial-Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346(22):1685–91.
Ortqvist E, Björk E, Wallensteen M, Ludvigsson J, Aman J, Johansson C, Forsander G, Lindgren F, Berglund L, Bengtsson M, Berne C, Persson B, Karlsson FA. Temporary preservation of beta-cell function by diazoxide treatment in childhood type 1 diabetes. Diabetes Care. 2004;27(9):2191–7.
Elliott RB, Chase HP. Prevention or delay of type 1 (insulin-dependent) diabetes mellitus in children using nicotinamide. Diabetologia. 1991;34(5):362–5.
Gale EA, Bingley PJ, Emmett CL, Collier T, European Nicotinamide Diabetes Intervention Trial (ENDIT) Group. European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet. 2004;363(9413):925–31.
Ludvigsson J, Samuelsson U, Johansson C, Stenhammar L. Treatment with antioxidants at onset of type 1 diabetes in children: a randomized, double-blind placebo-controlled study. Diabetes Metab Res Rev. 2001;17(2):131–6.
Mayer-Davis EJ, Dabelea D, Crandell JL, Crume T, D’Agostino RB Jr, Dolan L, King IB, Lawrence JM, Norris JM, Pihoker C, The N. Nutritional factors and preservation of C-peptide in youth with recently diagnosed type 1 diabetes: SEARCH Nutrition Ancillary Study. Diabetes Care. 2013;36(7):1842–50.
Hyppönen E, Läärä E, Reunanen A, Järvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes; a birth-cohort study. Lancet. 2001;358:1500–3.
Zipitis CS, Akobeng AK. Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systematic review and meta-analysis. Arch Dis Child. 2008;93:512–7.
Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O’Garra A. 1α,25-Dihydroxyvitamin D3 has a direct effect on naive CD4+ T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974–80.
Mathieu C, Gysemans C, Giulietti A, Bouillon R. Vitamin D and diabetes. Diabetologia. 2005;48:1247–57.
Markus W, Kaupper T, Adler K, Foersch J, Bonifacio E, Ziegler AG. No effect of the 1α,25-dihydroxyvitamin D3 on β-cell residual function and insulin requirement in adults with new-onset type 1 diabetes. Diabetes Care. 2010;33(7):1443–8.
Bizzarri C, Pitocco D, Napoli N, Di Stasio E, Maggi D, Manfrini S, Suraci C, Cavallo MG, Cappa M, Ghirlanda G, Pozzilli P, IMDIAB Group. No protective effect of calcitriol on β-cell function in recent-onset type 1 diabetes. The IMDIAB XIII trial. Diabetes Care. 2010;33(9):1962–3.
Samuelsson U, Oikarinen S, Hyöty H, Ludvigsson J. Low zinc in drinking water is associated with the risk of type 1 diabetes in children. Pediatr Diabetes. 2011;12(3 Pt 1):156–64.
Ludvigsson J, Heding L, Liedén G, Marner B, Lernmark A. Plasmapheresis in the initial treatment of insulin-dependent diabetes mellitus in children. Br Med J (Clin Res Ed). 1983;286(6360):176–8.
Stiller CR, Laupacis A, Dupre J, Jenner MR, Keown PA, Rodger W, Wolfe BM. Cyclosporine for treatment of early type I diabetes: preliminary results. N Engl J Med. 1983;308(20):1226–7.
Kitagawa T, Ludvigsson J. No cyclosporin A to diabetic children. Diabetes Care. 1988;11(5):447.
Voltarelli JC, Couri CE, Stracieri AB, Oliveira MC, Moraes DA, Pieroni F, Coutinho M, Malmegrim KC, Foss-Freitas MC, Simões BP, Foss MC, Squiers E, Burt RK. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2007;297(14):1568–76.
Silverstein J, Maclaren N, Riley W, Spillar R, Radjenovic D, Johnson S. Immunosuppression with azathioprine and prednisone in recent-onset insulin-dependent diabetes mellitus. N Engl J Med. 1988;319(10):599–604.
Eisenbarth GS, Srikanta S, Jackson R, et al. Anti-thymocyte globulin and prednisone immunotherapy of recent onset type 1 diabetes mellitus. Diabetes Res. 1985;2:271–6.
Haller MJ, Gitelman SE, Gottlieb PA, Michels AW, Rosenthal SM, Shuster JJ, Zou B, Brusko TM, Hulme MA, Wasserfall CH, Mathews CE, Atkinson MA, Schatz DA. Anti-thymocyte globulin/G-CSF treatment preserves β cell function in patients with established type 1 diabetes. J Clin Invest. 2015;125(1):448–55.
Eisenbarth GS, Srikanta S, Rabinowe SL, Jackson RA, Ganda OP, Soeldner JS. Restoration of first phase insulin secretion by daily prednisone in two islet cell antibody positive non-diabetic individuals. Transplant Proc. 1986;18:805–8.
Ludvigsson J. Immunological aspects on IDDM in children. Indian J Pediatr. 1989;56(Suppl 1):S7–14.
Pocecco M, De Campo C, Cantoni L, Tedesco F, Panizon F. Effect of high doses intravenous IgG in newly diagnosed diabetic children. Helv Paediatr Acta. 1987;42(4):289–95.
Ludvigsson J, Samuelsson U, Ernerudh J, Johansson C, Stenhammar L, Berlin G. Photopheresis at onset of type 1 diabetes: a randomised, double blind, placebo controlled trial. Arch Dis Child. 2001;85(2):149–54.
Faresjö MK, Ernerudh J, Berlin G, Garcia J, Ludvigsson J. The immunological effect of photopheresis in children with newly diagnosed type 1 diabetes. Pediatr Res. 2005;58(3):459–66.
Ernerudh J, Ludvigsson J, Berlin G, Samuelsson U. Effect of photopheresis on lymphocyte population in children with newly diagnosed type 1 diabetes. Clin Diagn Lab Immunol. 2004;11(5):856–61.
Herold KC, Hagopian W, Auger JA, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–8.
Sherry N, Hagopian W, Ludvigsson J, Jain SM, Wahlen J, Ferry RJ Jr, Bode B, Aronoff S, Holland C, Carlin D, King KL, Wilder RL, Pillemer S, Bonvini E, Johnson S, Stein KE, Koenig S, Herold KC, Daifotis AG, Protege Trial Investigators. Teplizumab for treatment of type 1 diabetes (Protege study): 1-year results from a randomised, placebo-controlled trial. Lancet. 2011;378(9790):487–97.
Hagopian W, Ferry RJ Jr, Sherry N, Carlin D, Bonvini E, Johnson S, Stein KE, Koenig S, Daifotis AG, Herold KC, Ludvigsson J, Protégé Trial Investigators. Teplizumab preserves C-peptide in recent-onset type 1 diabetes: two-year results from the randomized, placebo-controlled Protégé trial. Diabetes. 2013;62(11):3901–8.
Herold KC, Gitelman SE, Ehlers MR, Gottlieb PA, Greenbaum CJ, Hagopian W, Boyle KD, Keyes-Elstein L, Aggarwal S, Phippard D, Sayre PH, McNamara J, Bluestone JA, AbATE Study Team. Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes. 2013;62(11):3766–74.
Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin JM, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach JF, Pipeleers D, Chatenoud L. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352(25):2598–608.
Keymeulen B, Walter M, Mathieu C, et al. Four-year metabolic outcome of a randomised controlled CD3-antibody trial in recent-onset type 1 diabetic patients depends on their age and baseline residual β-cell mass. Diabetologia. 2010;53:614–23.
Aronson R, Gottlieb PA, Christiansen JS, Donner TW, Bosi E, Bode BW, et al. Low-dose otelixizumab anti-CD3 monoclonal antibody DEFEND-1 study: results of the randomized phase III study in recent-onset human type 1 diabetes. Diabetes Care. 2014;37(10):2746–54.
Keymeulen B, Candon S, Fafi-Kremer S, Ziegler A, Leruez-Ville M, Mathieu C, Vandemeulebroucke E, Walter M, Crenier L, Thervet E, Legendre C, Pierard D, Hale G, Waldmann H, Bach JF, Seigneurin JM, Pipeleers D, Chatenoud L. Transient Epstein–Barr virus reactivation in CD3 monoclonal antibody-treated patients. Blood. 2010;115(6):1145–55. doi:10.1182/blood-2009-02-204875. (Epub 2009 Dec 9).
Mastrandrea L, Yu J, Behrens T, Buchlis J, Albini C, Fourtner S, Quattrin T. Etanercept treatment in children with new-onset type 1 diabetes: pilot randomized, placebo-controlled, double-blind study. Diabetes Care. 2009;32(7):1244–9.
Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, Gottlieb PA, Marks JB, McGee PF, Moran AM, Raskin P, Rodriguez H, Schatz DA, Wherrett D, Wilson DM, Lachin JM, Skyler JS, Type 1 Diabetes TrialNet Anti-CD20 Study Group. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361(22):2143–52.
Ruderman EM, Richard PM. The evolving clinical profile of abatacept (CTLA4–Ig): a novel co-stimulatory modulator for the treatment of rheumatoid arthritis. Arthritis Res Therapy. 2005;7(Suppl 2):S21–5.
Bluestone JA, St. Clair EW, Turka LA. CTLA4Ig: bridging the basic immunology with clinical application. Immunity. 2006;24:233–8.
Orban T, Bundy B, Becker DJ, The Type 1 Diabetes TrialNet Abatacept Study Group, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised double-masked controlled trial. Lancet. 2011;378(9789):412–9.
Rigby MR, Harris KM, Pinckney A, DiMeglio LA, Rendell MS, Felner EI, Dostou JM, Gitelman SE, Griffin KJ, Tsalikian E, Gottlieb PA, Greenbaum CJ, Sherry NA, Moore WV, Monzavi R, Willi SM, Raskin P, Keyes-Elstein L, Long SA, Kanaparthi S, Lim N, Phippard D, Soppe CL, Fitzgibbon ML, McNamara J, Nepom GT, Ehlers MR. Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients. J Clin Invest. 2015;125(8):3285–96. doi:10.1172/JCI81722.
Faustman DL, Wang L, Okubo Y, Burger D, Ban L, Man G, Zheng H, Schoenfeld D, Pompei R, Avruch J, Nathan DM. Proof-of-concept, randomized, controlled clinical trial of Bacillus-Calmette–Guerin for treatment of long-term type 1 diabetes. PLoS One. 2012;7(8):e41756. doi:10.1371/journal.pone.0041756. (Epub 2012 Aug 8).
Allen HF, Klingensmith GJ, Jensen P, Simoes E, Hayward A, Chase HP. Effect of bacillus Calmette–Guerin vaccination on new-onset type 1 diabetes. A randomized clinical study. Diabetes Care. 1999;22(10):1703–7.
EURODIAB Substudy 2 Study Group. [No authors listed]. Infections and vaccinations as risk factors for childhood type I (insulin-dependent) diabetes mellitus: a multicentre case-control investigation. Diabetologia. 2000;43(1):47–53.
Vaarala O. The role of the gut in beta-cell autoimmunity and type 1 diabetes: a hypothesis. Pediatr Diabetes. 2000;1(4):217–25.
Vaarala O. Is it dietary insulin? Ann NY Acad Sci. 2006;1079:350–9.
Calcinaro F, Dionisi S, Marinaro M, et al. Oral probiotic administration induces interleukin-10 production and prevents spontaneous autoimmune diabetes in the non-obese diabetic mouse. Diabetologia. 2005;48:1565–75.
Simonen-Tikka ML, Hiekka AK, Klemola P, Poussa T, Ludvigsson J, Korpela R, Vaarala O, Roivainen M. Early human enterovirus infections in healthy Swedish children participating in the PRODIA pilot study. J Med Virol. 2012;84(6):923–30.
Elias D, Markovits D, Reshef T, van der Zee R, Cohen IR. Induction and therapy of autoimmune diabetes in the non-obese diabetic (NOD/Lt) mouse by a 65-kDa heat shock protein. Proc Natl Acad Sci USA. 1990;87(4):1576–80.
Raz I, Elias D, Avron A, Tamir M, Metzger M, Cohen IR. Beta-cell function in new-onset type 1 diabetes and immunomodulation with a heat-shock protein peptide (DiaPep277): a randomised, double-blind, phase II trial. Lancet. 2001;358(9295):1749–53.
Schloot NC, Meierhoff G, Lengyel C, Vándorfi G, Takács J, Pánczél P, Barkai L, Madácsy L, Oroszlán T, Kovács P, Sütö G, Battelino T, Hosszufalusi N, Jermendy G. Effect of heat shock protein peptide DiaPep277 on beta-cell function in paediatric and adult patients with recent-onset diabetes mellitus type 1: two prospective, randomized, double-blind phase II trials. Diabetes Metab Res Rev. 2007;23(4):276–85.
Huurman VA, van der Meide PE, Duinkerken G, Willemen S, Cohen IR, Elias D, Roep BO. Immunological efficacy of heat shock protein 60 peptide DiaPep277 therapy in clinical type I diabetes. Clin Exp Immunol. 2008;152(3):488–97.
Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775.
Grinberg-Bleyer Y, Baeyens A, You S, Elhage R, Fourcade G, Gregoire S, et al. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J Exp Med. 2010;207:1871.
Long SA, Rieck M, Sanda S, Bollyky JB, Samuels PL, Goland R, et al. Rapamycin/IL-2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently impairs beta-cell function. Diabetes. 2012;61:2340.
Long SA, Rieck M, Sanda S, Bollyky JB, Samuels PL, Goland R, Ahmann A, Rabinovitch A, Aggarwal S, Phippard D, Turka LA, Ehlers MR, Bianchine PJ, Boyle KD, Adah SA, Bluestone JA, Buckner JH, Greenbaum CJ, Diabetes TrialNet and the Immune Tolerance Network. Rapamycin/IL-2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently impairs β-cell function. Diabetes. 2012;61(9):2340–8. doi:10.2337/db12-0049.
Simms MS, Scholfield DP, Jacobs E, et al. Ati-GnRH antibodies can induce castrate levels of testosterone in patients with advanced prostate cancer. Br J Cancer. 2000;83:443–6.
Johansen PAC, et al. Direct intralymphatic injection of peptide vaccines enhances immunogenicity. Eur J Immunol. 2005;35(2):568–74.
Shoda LK, Young DL, Ramanujan S, Whiting CC, Atkinson MA, Bluestone JA, et al. A comprehensive review of interventions in the NOD mouse and implications for translation. Immunity. 2005;23(2):115–26.
Boettler T, von Herrath M. Type 1 diabetes vaccine development: animal models vs. humans. Hum Vaccin. 2011;7(1):19–26.
Staeva-Vieira T, Peakman M, von Herrath M. Translational mini-review series on type 1 diabetes: immune-based therapeutic approaches for type 1 diabetes. Clin Exp Immunol. 2007;148(1):17–31.
Quinn A, McInerney B, Reich EP, Kim O, Jensen KP, Sercarz EE. Regulatory and effector CD4 T cells in nonobese diabetic mice recognize overlapping determinants on glutamic acid decarboxylase and use distinct V beta genes. J Immunol. 2001;166(5):2982–91.
Homann D, Holz A, Bot A, Coon B, Wolfe T, Petersen J, et al. Autoreactive CD4+ T cells protect from autoimmune diabetes via bystander suppression using the IL-4/Stat6 pathway. Immunity. 1999;11(4):463–72.
Peakman M, von Herrath M. Antigen-specific immunotherapy for type 1 diabetes: maximizing the potential. Diabetes. 2010;59(9):2087–93.
Tisch R, Wang B, Serreze DV. Induction of glutamic acid decarboxylase 65-specific Th2 cells and suppression of autoimmune diabetes at late stages of disease is epitope dependent. J Immunol. 1999;163(3):1178–87.
Ludvigsson J. Adequate doses of autoantigen administered using the appropriate route may create tolerance and stop autoimmunity. Diabetologia. 2009;52(1):175–6.
Martens H, Goxe B, Geenen V. The thynmic repertoire of neuroendocrine-related self-antigens: physiological implications in T-cell life and death. Immunol. Today. 1996;17:312–7.
Kyewski B, Klein L. A central role for central tolerance. Annu Rev Immunol. 2006;24:571–606.
Fan Y, Rudert WA, Grupillo M, He J, Sisino G, Trucco M. Thymus-specific deletion of insulin induces autoimmune diabetes. EMBO J. 2009;28(18):2812–24.
Skyler JS, Krischer JP, Wolfsdorf J, Cowie C, Palmer JP, Greenbaum C, et al. Effects of oral insulin in relatives of patients with type 1 diabetes: the Diabetes Prevention Trial-Type 1. Diabetes Care. 2005;28(5):1068–76.
Näntö-Salonen K, Kupila A, Simell S, Siljander H, Salonsaari T, Hekkala A, Korhonen S, Erkkola R, Sipilä JI, Haavisto L, Siltala M, Tuominen J, Hakalax J, Hyöty H, Ilonen J, Veijola R, Simell T, Knip M, Simell O. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet. 2008;372(9651):1746–55.
Orban T, Farkas K, Jalahej H, Kis J, Treszl A, Falk B, Reijonen H, Wolfsdorf J, Ricker A, Matthews JB, Tchao N, Sayre P, Bianchine P. Autoantigen-specific regulatory T cells induced in patients with type 1 diabetes mellitus by insulin B-chain immunotherapy. J Autoimmun. 2010;34(4):408–15.
Thrower SL, James L, Hall W, Green KM, Arif S, Allen JS, Van-Krinks C, Lozanoska-Ochser B, Marquesini L, Brown S, Wong FS, Dayan CM, Peakman M. Proinsulin peptide immunotherapy in type 1 diabetes: report of a first-in-man phase I safety study. Clin Exp Immunol. 2009;155(2):156–65.
Baekkeskov S, Nielsen JH, Marner B, Bilde T, Ludvigsson J, Lernmark A. Autoantibodies in newly diagnosed diabetic children immunoprecipitate human pancreatic islet cell proteins. Nature. 1982;298(5870):167–9.
Agardh CD, Cilio CM, Lethagen A, et al. Clinical evidence for the safety of GAD65 immunomodulation in adult-onset autoimmune diabetes. J Diabetes Complications. 2005;19:238–46.
Agardh C-D, Lynch K, Palmér M, Link K, et al. GAD65 vaccination significantly reduces insulin dependence at five years follow-up in a dose escalating study in adult-onset autoimmune diabetes patients. Diabetologia. 2008;51(Suppl. 1):S230.
Ludvigsson J, Faresjo M, Hjorth M, Axelsson S, Cheramy M, Pihl M, et al. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med. 2008;359(18):1909–20.
Ludvigsson J, Hjorth M, Chéramy M, Axelsson S, et al. Extended evaluation of the safety and efficacy of GAD treatment of children and adolescents with recent-onset type 1 diabetes: a randomised controlled trial. Diabetologia. 2011;54(3):634–40.
Ludvigsson J, Krisky D, Casas R, Battelino T, et al. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N Engl J Med. 2012;366(5):433–42.
Ludvigsson J, Chéramy M, Axelsson S, Pihl M, Akerman L, Casas R. GAD-treatment of children and adolescents with recent-onset Type 1 diabetes preserves residual insulin secretion after 30 months. For the clinical GAD-study group in Sweden. Diabetes Metab Res Rev. 2013.
Chéramy M, Skoglund C, Johansson I, Ludvigsson J, Hampe CS, Casas R. GAD-alum treatment in patients with type 1 diabetes and the subsequent effect on GADA IgG subclass distribution, GAD(65) enzyme activity and humoral response. Clin Immunol. 2010;137(1):31–40. doi:10.1016/j.clim.2010.06.001.
Axelsson S, Chéramy M, Hjorth M, Pihl M, et al. Long-lasting immune responses 4 years after GAD-alum treatment in children with type 1 diabetes. PLoS One. 2011;6(12):e29008.
Hjorth M, Axelsson S, Rydén A, Faresjö M, Ludvigsson J, et al. GAD-alum treatment induces GAD65-specific CD4+ CD25 high FOXP3+ cells in type 1 diabetic patients. Clin Immunol. 2011;138(1):117–26.
Wherrett DK, Bundy B, Becker DJ, et al. Antigen-based therapy with glutamicacid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet. 2011;378(9788):319–27.
von Herrath MG, Whitton JL. DNA vaccination to treat autoimmune diabetes. Ann Med. 2000;32(5):285–92.
Coon B, An LL, Whitton JL, von Herrath MG. DNA immunization to prevent autoimmune diabetes. J Clin Invest. 1999;104(2):189–94.
Jun HS. Regeneration of pancreatic beta cells. Front Biosci. 2008;1(13):6170–82.
Rother KI, Spain LM, Wesley RA, Digon BJ 3rd, Baron A, Chen K, Nelson P, Dosch HM, Palmer JP, Brooks-Worrell B, Ring M, Harlan DM. Effects of exenatide alone and in combination with daclizumab on beta-cell function in long-standing type 1 diabetes. Diabetes Care. 2009;32(12):2251–7.
Dungan KM, Buse JB, Ratner RE. Effects of therapy in type 1 and type 2 diabetes mellitus with a peptide derived from islet neogenesis associated protein (INGAP). Diabetes Metab Res Rev. 2009;25(6):558–65.
Pickersgill LM, Mandrup-Poulsen TR. The anti-interleukin-1 in type 1 diabetes action trial–background and rationale. Diabetes Metab Res Rev. 2009;25(4):321–4 (Review).
Moran A, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, Greenbaum CJ, Herold KC, Marks JB, Raskin P, Sanda S, Schatz D, Wherrett DK, Wilson DM, Krischer JP, Skyler JS, Type 1 Diabetes TrialNet Canakinumab Study Group, Pickersgill L, de Koning E, Ziegler AG, Böehm B, Badenhoop K, Schloot N, Bak JF, Pozzilli P, Mauricio D, Donath MY, Castaño L, Wägner A, Lervang HH, Perrild H, Mandrup-Poulsen T, AIDA Study Group, Pociot F, Dinarello CA. Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet. 2013;381(9881):1905–15.
Acknowledgments
In addition to the author, the Linköping Diabetes Immune Intervention study group consists of Assistant Professor Rosaura Casas, a number of postdoctoral researchers (currently Sri Elluru, Hugo Barcenilla, Saubashya Sur, Betatriz Iglesias) and PhD students including Linda Åkerman.
We are grateful to excellent technical assistance from Ingela Johansson and Gosia Smolinska, and from research nurses Eva Isacson and AnnMarie Sandström. All pediatricians involved in our studies are also gratefully acknowledged.
Our studies on immune intervention have been generously supported by Barndiabetesfonden (Swedish Child Diabetes Foundation), Swedish Research Council, Research Council of Southeast Sweden (FORSS), and the mechanistic studies are supported by the Juvenile Diabetes Research Foundation (JDFR).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding and disclosures
Diamyd Medical sponsored the phase II/III GAD trials and has also given unrestricted financial support for the investigator-initiated mechanistic studies connected to these trials. Honoraria for lectures have been received from NovoNordisk, Lilly and Sanofi Aventis., and as a member of Advisory Boards of LifeScan and DebioPharm.
Rights and permissions
About this article
Cite this article
Ludvigsson, J. Therapies to Preserve β-Cell Function in Type 1 Diabetes. Drugs 76, 169–185 (2016). https://doi.org/10.1007/s40265-015-0511-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-015-0511-x