Abstract
Background and Objective
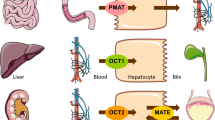
Metformin is contraindicated in patients with renal impairment; however, there is poor adherence to current dosing guidelines. In addition, the pharmacokinetics of metformin in patients with significant renal impairment are not well described. The aims of this study were to investigate factors influencing the pharmacokinetic variability, including variant transporters, between healthy subjects and patients with type 2 diabetes mellitus (T2DM) and to simulate doses of metformin at varying stages of renal function.
Methods
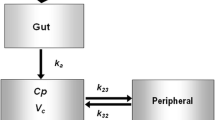
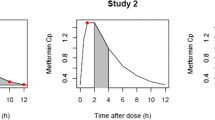
Plasma concentrations of metformin were pooled from three studies: patients with T2DM (study A; n = 120), healthy Caucasian subjects (study B; n = 16) and healthy Malaysian subjects (study C; n = 169). A population pharmacokinetic model of metformin was developed using NONMEM® version VI for both the immediate-release (IR) formulation and the extended-release (XR) formulation of metformin. Total body weight (TBW), lean body weight (LBW), creatinine clearance (CLCR; estimated using TBW and LBW) and 57 single-nucleotide polymorphisms (SNPs) of metformin transporters (OCT1, OCT2, OCT3, MATE1 and PMAT) were investigated as potential covariates. A nonparametric bootstrap (n = 1,000) was used to evaluate the final model. This model was used to simulate 1,000 concentration–time profiles for doses of metformin at each stage of renal impairment to ensure metformin concentrations do not exceed 5 mg/l, the proposed upper limit.
Results
Creatinine clearance and TBW were clinically and statistically significant covariates with the apparent clearance and volume of distribution of metformin, respectively. None of the 57 SNPs in transporters of metformin were significant covariates. In contrast to previous studies, there was no effect on the pharmacokinetics of metformin in patients carrying the reduced function OCT1 allele (R61C, G401S, 420del or G465R). Dosing simulations revealed that the maximum daily doses in relation to creatinine clearance to prescribe to patients are 500 mg (15 ml/min), 1,000 mg (30 ml/min), 2,000 mg (60 ml/min) and 3,000 mg (120 ml/min), for both the IR and XR formulations.
Conclusion
The population model enabled doses of metformin to be simulated for each stage of renal function, to ensure the concentrations of metformin do not exceed 5 mg/l. However, the plasma concentrations of metformin at these dosage levels are still quite variable and monitoring metformin concentrations may be of value in individualising dosage. This study provides confirmatory data that metformin can be used, with appropriate dosage adjustment, in patients with renal impairment.



Similar content being viewed by others
References
World Health Organization. Diabetes fact sheet no. 312. 2012. http://www.who.int/mediacentre/factsheets/fs312/en/index.html. Accessed 19 Feb 2012
Hundal RS, Krssak M, Dufour S, et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49(12):2063–9.
Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):854–65.
Diabex tablets. Carole Park, Australia: Alphapharm Pty Ltd; 2012
Bruijstens LA, van Luin M, Buscher-Jungerhans PM, et al. Reality of severe metformin-induced lactic acidosis in the absence of chronic renal impairment. Neth J Med. 2008;66(5):185–90.
Graham GG, Punt J, Arora M, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50(2):81–98.
Tzvetkov MV, Vormfelde SV, Balen D, et al. The effects of genetic polymorphisms in the organic cation transporters OCT1, OCT2, and OCT3 on the renal clearance of metformin. Clin Pharmacol Ther. 2009;86(3):299–306.
Zhou M, Xia L, Wang J. Metformin transport by a newly cloned proton-stimulated organic cation transporter (plasma membrane monoamine transporter) expressed in human intestine. Drug Metab Dispos. 2007;35(10):1956–62.
Bardin C, Nobecourt E, Larger E, et al. Population pharmacokinetics of metformin in obese and non-obese patients with type 2 diabetes mellitus. Eur J Clin Pharmacol. 2012;68(6):961–8.
Tucker GT, Casey C, Phillips PJ, et al. Metformin kinetics in healthy subjects and in patients with diabetes mellitus. Br J Clin Pharmacol. 1981;12(2):235–46.
Timmins P, Donahue S, Meeker J, et al. Steady-state pharmacokinetics of a novel extended-release metformin formulation. Clin Pharmacokinet. 2005;44(7):721–9.
Shu Y, Brown C, Castro RA, et al. Effect of genetic variation in the organic cation transporter 1, OCT1, on metformin pharmacokinetics. Clin Pharmacol Ther. 2008;83(2):273–80.
Sambol NC, Chiang J, O’Conner M, et al. Pharmacokinetics and pharmacodynamics of metformin in healthy subjects and patients with noninsulin-dependent diabetes mellitus. J Clin Pharmacol. 1996;36(11):1012–21.
Hong Y, Rohatagi S, Habtemariam B, et al. Population exposure-response modeling of metformin in patients with type 2 diabetes mellitus. J Clin Pharmacol. 2008;48(6):696–707.
Christensen MM, Brasch-Andersen C, Green H, et al. The pharmacogenetics of metformin and its impact on plasma metformin steady-state levels and glycosylated hemoglobin A1c. Pharmacogenet Genomics. 2011;21(12):837–50.
Lalau JD, Lemaire-Hurtel AS, Lacroix C. Establishment of a database of metformin plasma concentrations and erythrocyte levels in normal and emergency situations. Clin Drug Investig. 2011;31(6):435–8.
Duong JK, Roberts DM, Furlong TJ, et al. Metformin therapy in patients with chronic kidney disease. Diabetes Obes Metab. 2012;15(10):963–5.
Zarghi A, Foroutan SM, Shafaati A, et al. Rapid determination of metformin in human plasma using ion-pair HPLC. J Pharm Biomed Anal. 2003;31(1):197–200.
Boeckman A, Sheiner A, Beal S. NONMEM 6. Ellicott City: GloboMax, ICON Development Solutions; 2007.
Wang Y. Derivation of various NONMEM estimation methods. J Pharmacokinet Pharmacodyn. 2007;34(5):575–93.
Cullberg M, Eriksson UG, Larsson M, et al. Population modelling of the effect of inogatran, at thrombin inhibitor, on ex vivo coagulation time (APTT) in healthy subjects and patients with coronary artery disease. Br J Clin Pharmacol. 2001;51(1):71–9.
Janmahasatian S, Duffull SB, Chagnac A, et al. Lean body mass normalizes the effect of obesity on renal function. Br J Clin Pharmacol. 2008;65(6):964–5.
Janmahasatian S, Duffull SB, Ash S, et al. Quantification of lean bodyweight. Clin Pharmacokinet. 2005;44(10):1051–65.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41.
Hooker AC, Staatz CE, Karlsson MO. Conditional weighted residuals (CWRES): a model diagnostic for the FOCE method. Pharm Res. 2007;24(12):2187–97.
Brendel K, Comets E, Laffont C, et al. Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide. Pharm Res. 2006;23(9):2036–49.
Henderson AR. The bootstrap: a technique for data-driven statistics. Using computer-intensive analyses to explore experimental data. Clin Chim Acta. 2005;359(1–2):1–26.
Wigginton JE, Cutler DJ, Abecasis GR. A note on exact tests of Hardy–Weinberg equilibrium. Am J Hum Genet. 2005;76(5):887–93.
Roos JF, Kirkpatrick CM, Tett SE, et al. Development of a sufficient design for estimation of fluconazole pharmacokinetics in people with HIV infection. Br J Clin Pharmacol. 2008;66(4):455–66.
Lalau JD, Lacroix C. Measurement of metformin concentration in erythrocytes: clinical implications. Diabetes Obes Metab. 2003;5(2):93–8.
Sambol NC, Chiang J, Lin ET, et al. Kidney function and age are both predictors of pharmacokinetics of metformin. J Clin Pharmacol. 1995;35(11):1094–102.
Bonate PL. The effect of collinearity on parameter estimates in nonlinear mixed effect models. Pharm Res. 1999;16(5):709–17.
Bricker NS, Morrin PA, Kime SW Jr. The pathologic physiology of chronic Bright’s disease. An exposition of the “intact nephron hypothesis”. Am J Med. 1960;28:77–98.
Nies AT, Koepsell H, Winter S, et al. Expression of organic cation transporters OCT1 (SLC22A1) and OCT3 (SLC22A3) is affected by genetic factors and cholestasis in human liver. Hepatology. 2009;50(4):1227–40.
Aoki M, Terada T, Kajiwara M, et al. Kidney-specific expression of human organic cation transporter 2 (OCT2/SLC22A2) is regulated by DNA methylation. Am J Physiol Ren Physiol. 2008;295(1):F165–70.
Kamber N, Davis WA, Bruce DG, et al. Metformin and lactic acidosis in an Australian community setting: the Fremantle Diabetes Study. Med J Aust. 2008;188(8):446–9.
Nye HJ, Herrington WG. Metformin: the safest hypoglycaemic agent in chronic kidney disease? Nephron Clin Pract. 2011;118(4):c380–3.
Rossi S. Australian medicines handbook 2011. Adelaide: Australian Medicines Handbook Pty Ltd; 2011.
Frid A, Sterner GN, Londahl M, et al. Novel assay of metformin levels in patients with type 2 diabetes and varying levels of renal function: clinical recommendations. Diabetes Care. 2010;33(6):1291–3.
Kamber N, Davis WA, Bruce DG, et al. Metformin and lactic acidosis in an Australian community setting: the Fremantle Diabetes Study. Med J Aust. 2008;188(8):446–9.
Briet C, Saraval-Gross M, Kajbaf F, et al. Erythrocyte metformin levels in patients with type 2 diabetes and varying severity of chronic kidney disease. Clin Kidney J. 2012;5(1):65–7.
Acknowledgements
The authors would like to thank Prof. Kathleen Giacomini for advice on the selection of SNPs in metformin transporters and Dr. Pavel Bitter for the analyses of SNPs. Funding for this study was provided by the NH&MRC Programme Grant 568612, Australian Research Council Grant LP 0990670, and St Vincent’s Clinic Foundation Sister Mary Bernice Research Grant.
Conflicts of interest
Peter Timmins is a salaried employee of Bristol-Myers Squibb, which is involved in the development and marketing of products containing metformin. Apart from the salary of Dr. Timmins, Bristol-Myers Squibb made no other payments in the support of this work. All other authors declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Duong, J.K., Kumar, S.S., Kirkpatrick, C.M. et al. Population Pharmacokinetics of Metformin in Healthy Subjects and Patients with Type 2 Diabetes Mellitus: Simulation of Doses According to Renal Function. Clin Pharmacokinet 52, 373–384 (2013). https://doi.org/10.1007/s40262-013-0046-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-013-0046-9