Abstract
Background and Objective
Pulmonary arterial hypertension (PAH) is an intractable and rare disease and the accumulation of clinical evidence under real-world setting is needed. A post-marketing surveillance for the endothelin receptor antagonist ambrisentan (Volibris tablet) has been conducted by all-case investigation since September 2010. This paper is an interim report on the safety and efficacy of ambrisentan in 702 patients with PAH.
Methods
PAH patients aged 15 years or older were subjected to the analysis. The safety analysis by overall cases or stratification of patient backgrounds and the efficacy analysis were investigated.
Results
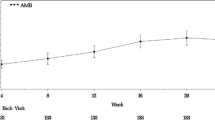
Regarding patient characteristics, the 702 patients subjected to safety analysis included 543 (77.4%) women and 546 (77.8%) patients at WHO functional class II/III. The mean observational time was 392.7 days. A total of 324 adverse drug reaction (ADR) occurred in 204 (29.1%) patients. Common ADRs (≥ 2%) included anemia (4.6%), peripheral edema (4.1%), headache (3.6%), edema and face edema (2.6% each), abnormal hepatic function (2.3%), and epistaxis (2.1%). There were 82 serious ADRs occurring in 44 (6.3%) patients (385 serious adverse events in 184 (26.2%) patients). Although 11 (1.6%) interstitial lung disease (ILD) cases were reported, all were observed in patients with disease that may contribute to ILD and therefore it is difficult to assess if ambrisentan was associated with these events. There was no difference in safety in relation to the presence/absence of connective tissue disease-related PAH (CTD-PAH) or combination therapy. Among 677 patients subjected to efficacy analysis, those in whom hemodynamic status was determined before and after treatment showed improvement in the mean pulmonary arterial pressure and pulmonary vascular resistance after treatment.
Conclusion
The interim results showed safety consistent with the known profile of ambrisentan in terms of the types and frequencies of ADRs in patients with PAH in real clinical practice, in comparison with previous clinical trials in Japan and the rest of the world. Thus, these results provided another corroboration of the tolerability of ambrisentan and we continue to monitor proper use information via the post-marketing surveillance to ensure any new safety signals are identified in a timely manner (ClinTrial.gov: NCT01406327).


Similar content being viewed by others
References
D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115(5):343–9.
Farber HW, Miller DP, Poms AD, et al. Five-Year outcomes of patients enrolled in the REVEAL Registry. CHEST. 2015;148(4):1043–54.
Akagi S, Nakamura K, Matsubara H, et al. Epoprostenol therapy for pulmonary arterial hypertension. Acta Med Okayama. 2015;69(3):129–36.
Tamura Y, Channick RN. New paradigm for pulmonary arterial hypertension treatment. Curr Opin Pulm Med. 2016;22(5):429–33.
Humbert M, Ghofrani HA. The molecular targets of approved treatments for pulmonary arterial hypertension. Thorax. 2016;71(1):73–83.
Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37(1):67–119.
Peacock AJ, Zamboni W, Vizza CD. Ambrisentan for the treatment of adults with pulmonary arterial hypertension: a review. Curr Med Res Opin. 2015;31(9):1793–807.
McGoon MD, Frost ME, Oudiz RJ. Ambrisentan therapy in patients with pulmonary arterial hypertension who discontinued bosentan or sitaxsentan due to liver function test abnormalities. CHEST. 2009;135:122–9.
Hakamata A, Odagiri K, Miyakawa S, et al. Pharmacokinetic and pharmacodynamic comparison of sildenafil-bosentan and sildenafil-ambrisentan combination therapies for pulmonary hypertension. Clin Transl Sci. 2016;9(1):29–35.
Yoshida S, Shirato K, Shimamura R, et al. Efficacy, safety, and pharmacokinetics of ambrisentan in Japanese adults with pulmonary arterial hypertension. Curr Med Res Opin. 2011;27(9):1827–34.
Galiè N, Olschewski H, Oudiz RJ, et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 2008;117:3010–9.
Oudiz RJ, Galiè N, Olschewski H, et al. Long-term ambrisentan therapy for the treatment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:1971–81.
Yoshida S, Shirato K, Shimamura R, et al. Long-term safety and efficacy of ambrisentan in Japanese adults with pulmonary arterial hypertension. Curr Med Res Opin. 2012;28:1069–76.
Traiger GL. Pulmonary arterial hypertension. Crit Care Nurs Q. 2007;30(1):20–43.
Vachiéry JL, Hoeper MM, Peacock AJ, et al. Ambrisentan use for pulmonary arterial hypertension in a post-authorization drug registry: The VOLibris tracking study. J Heart Lung Transplant. 2017;36(4):399–406.
Sasayama S, Kunieda T, Tomoike H, et al. Effects of the endothelin receptor antagonist bosentan on hemodynamics, symptoms and functional capacity in Japanese patients with severe pulmonary hypertension. Circ J. 2005;69:131–7.
Tahara N, Dobashi H, Fukuda K, et al. Efficacy and safety of a novel endothelin receptor antagonist, macitentan, in Japanese patients with pulmonary arterial hypertension. Circ J. 2016;80(6):1478–83.
Raghu G, Behr J, Brown KK, Egan JJ, et al. Treatment of idiopathic pulmonary fibrosis with ambrisentan: a parallel, randomized trial. Ann Intern Med. 2013;158(9):641–9.
Efficacy and safety of riociguat in patients with symptomatic pulmonary hypertension (PH) associated with idiopathic interstitial pneumonias (IIP) (RISE-IIP)” (NCT02138825). In: Clinicaltrial.gov. https://clinicaltrials.gov/ct2/show/results/NCT02138825?term=riociguat&cond=IIP&rank=1. Accessed Oct 2017.
King TE Jr, Brown KK, Raghu G, et al. BUILD-3: a randomized, controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184(1):92–9.
Raghu G, Million-Rousseau R, Morganti A, et al. Macitentan for the treatment of idiopathic pulmonary fibrosis: the randomised controlled MUSIC trial and Supplementary material. Eur Respir J. 2013;42(6):1622–32.
Sobanski V, Launay D, Hachulla E, et al. Current approaches to the treatment of systemic-sclerosis-associated pulmonary arterial hypertension (SSc-PAH). Curr Rheumatol Rep. 2016;18(2):10.
Ichimura Y, Tanabe N, Tatsumi K. The progress of diagnosis and therapy in pulmonary hypertension due to interstitial pneumonia. Ann Jpn Respir Soc. 2014;3(4):492–7 (in Japanese).
Seeger W, Adir Y, Barberà JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol. 2013;62(25 Suppl):D109–16.
Nakanishi N, Ando T, Ueda H, et al. Guidelines for Treatment of Pulmonary Hypertension (JCS2012. 2nd edn. The Japanese Circulation Society; 2012 (in Japanese)
Galiè N, Barberà JA, Frost AE, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med. 2015;373:834–44.
Hatano M, Yamada H, Fukuda K, et al. Effects of the endothelin receptor antagonist bosentan on hemodynamics and exercise capacity in Japanese patients with mildly symptomatic pulmonary arterial hypertension. Heart Vessels. 2015;30:798–804.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Funding GlaxoSmithKline K.K. funded this surveillance. Trademarks are owned by or licensed to the GSK group of companies
Conflict of interest
Tomohiko Takahashi and Satoru Hayata, Akihiro Kobayashi, Yuna Onaka, Takeshi Ebihara and Terufumi Hara are employees of GlaxoSmithKline K.K and Akihiro Kobayashi,Yuna Onaka, Takeshi Ebihara, and Terufumi Hara hold shares in the company. This investigation was conducted by GlaxoSmithKline K.K. We ensure that all potential conflicts of interest were disclosed.
Ethical approval
This investigation was conducted in accordance with the Japanese Good Post-Marketing Study Practice (GPSP, Ordinance of Ministry of Health, Labour and Welfare No. 171 of December 20, 2004). Informed consent was not required from all individual participants included in this survey according to this GPSP. The agreement for publication on this survey was obtained from all the participating institutions. The rules of personal data confidentiality were fully respected.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Takahashi, T., Hayata, S., Kobayashi, A. et al. Surveillance on The Safety and Efficacy of Ambrisentan (Volibris Tablet 2.5 mg) in Patients with Pulmonary Arterial Hypertension in Real Clinical Practice: Post-marketing Surveillance (Interim Analysis Report). Clin Drug Investig 38, 219–229 (2018). https://doi.org/10.1007/s40261-017-0602-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-017-0602-4