Abstract
Purpose of Review
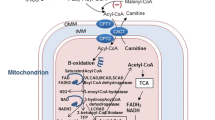
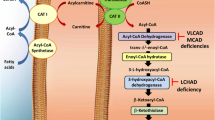
This review focuses on advances made in the past 3 years with regards to understanding the mitochondrial fatty acid oxidation (FAO) pathway, the pathophysiological ramifications of genetic lesions in FAO enzymes, and emerging therapies for FAO disorders.
Recent Findings
FAO has now been recognized to play a key energetic role in pulmonary surfactant synthesis, T-cell differentiation and memory, and the response of the proximal tubule to kidney injury. Patients with FAO disorders may face defects in these cellular systems as they age. Aspirin, statins, and nutritional supplements modulate the rate of FAO under normal conditions and could be risk factors for triggering symptoms in patients with FAO disorders. Patients have been identified with mutations in the ACAD9 and ECHS1 genes, which may represent new FAO disorders. New interventions for long-chain FAODs are in clinical trials. Finally, post-translational modifications that regulate fatty acid oxidation protein activities have been characterized that represent important new therapeutic targets.
Summary
Recent research has led to a deeper understanding of FAO. New therapeutic avenues are being pursued that may ultimately cause a paradigm shift for patient care.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Yew Tan C, Virtue S, Murfitt S, Roberts LD, Phua YH, Dale M, et al. Adipose tissue fatty acid chain length and mono-unsaturation increases with obesity and insulin resistance. Sci Rep. 2015;5:18366. doi:10.1038/srep18366.
Spiekerkoetter U, Bastin J, Gillingham M, Morris A, Wijburg F, Wilcken B. Current issues regarding treatment of mitochondrial fatty acid oxidation disorders. J Inherit Metab Dis. 2010;33(5):555–61. doi:10.1007/s10545-010-9188-1.
Houten SM, Violante S, Ventura FV, Wanders RJ. The biochemistry and physiology of mitochondrial fatty acid beta-oxidation and its genetic disorders. Annu Rev Physiol. 2016;78:23–44. doi:10.1146/annurev-physiol-021115-105045.
Vishwanath VA. Fatty acid beta-oxidation disorders: a brief review. Ann Neurosci. 2016;23(1):51–5. doi:10.1159/000443556.
Schiff M, Mohsen AW, Karunanidhi A, McCracken E, Yeasted R, Vockley J. Molecular and cellular pathology of very-long-chain acyl-CoA dehydrogenase deficiency. Mol Genet Metab. 2013;109(1):21–7. doi:10.1016/j.ymgme.2013.02.002.
Fletcher AL, Pennesi ME, Harding CO, Weleber RG, Gillingham MB. Observations regarding retinopathy in mitochondrial trifunctional protein deficiencies. Mol Genet Metab. 2012;106(1):18–24. doi:10.1016/j.ymgme.2012.02.015.
Guerra C, Koza RA, Walsh K, Kurtz DM, Wood PA, Kozak LP. Abnormal nonshivering thermogenesis in mice with inherited defects of fatty acid oxidation. J Clin Invest. 1998;102(9):1724–31.
Pascual F, Coleman RA. Fuel availability and fate in cardiac metabolism: a tale of two substrates. Biochim Biophys Acta. 2016;1860(10):1425–33. doi:10.1016/j.bbalip.2016.03.014.
Rui L. Energy metabolism in the liver. Compr Physiol. 2014;4(1):177–97. doi:10.1002/cphy.c130024.
van Hall G. The physiological regulation of skeletal muscle fatty acid supply and oxidation during moderate-intensity exercise. Sports Med. 2015;45(Suppl 1):S23–32. doi:10.1007/s40279-015-0394-8.
Goetzman ES, Alcorn JF, Bharathi SS, Uppala R, McHugh KJ, Kosmider B, et al. Long-chain acyl-CoA dehydrogenase deficiency as a cause of pulmonary surfactant dysfunction. J Biol Chem. 2014;289(15):10668–79. doi:10.1074/jbc.M113.540260.
Chiaranunt P, Ferrara JL, Byersdorfer CA. Rethinking the paradigm: how comparative studies on fatty acid oxidation inform our understanding of T cell metabolism. Mol Immunol. 2015;68(2 Pt C):564–74. doi:10.1016/j.molimm.2015.07.023.
Hackl A, Mehler K, Gottschalk I, Vierzig A, Eydam M, Hauke J, et al. Disorders of fatty acid oxidation and autosomal recessive polycystic kidney disease-different clinical entities and comparable perinatal renal abnormalities. Pediatr Nephrol. 2017;32(5):791–800. doi:10.1007/s00467-016-3556-5.
Stadler K, Goldberg IJ, Susztak K. The evolving understanding of the contribution of lipid metabolism to diabetic kidney disease. Curr Diab Rep. 2015;15(7):40. doi:10.1007/s11892-015-0611-8.
DiMauro S, DiMauro PM. Muscle carnitine palmityltransferase deficiency and myoglobinuria. Science. 1973;182(4115):929–31.
Otsubo C, Bharathi S, Uppala R, Ilkayeva OR, Wang D, McHugh K, et al. Long-chain acylcarnitines reduce lung function by inhibiting pulmonary surfactant. J Biol Chem. 2015; doi:10.1074/jbc.M115.655837.
Chegary M, Brinke H, Ruiter JP, Wijburg FA, Stoll MS, Minkler PE, et al. Mitochondrial long chain fatty acid beta-oxidation in man and mouse. Biochim Biophys Acta. 2009;1791(8):806–15. doi:10.1016/j.bbalip.2009.05.006.
Lundy CT, Shield JP, Kvittingen EA, Vinorum OJ, Trimble ER, Morris AA. Acute respiratory distress syndrome in long-chain 3-hydroxyacyl-CoA dehydrogenase and mitochondrial trifunctional protein deficiencies. J Inherit Metab Dis. 2003;26(6):537–41.
Gentili A, Iannella E, Masciopinto F, Latrofa ME, Giuntoli L, Baroncini S. Rhabdomyolysis and respiratory failure: rare presentation of carnitine palmityl-transferase II deficiency. Minerva Anestesiol. 2008;74(5):205–8.
Le Hir M, Dubach UC. Peroxisomal and mitochondrial beta-oxidation in the rat kidney: distribution of fatty acyl-coenzyme A oxidase and 3-hydroxyacyl-coenzyme A dehydrogenase activities along the nephron. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 1982;30(5):441–4. doi:10.1177/30.5.7200500.
Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest. 2009;119(5):1275–85. doi:10.1172/JCI37829.
Meir K, Fellig Y, Meiner V, Korman SH, Shaag A, Nadjari M, et al. Severe infantile carnitine palmitoyltransferase II deficiency in 19-week fetal sibs. Pediatric and developmental pathology : the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society. 2009;12(6):481–6. doi:10.2350/08-10-0548.1.
Giuliani E, Coppi F, Bertolotti V, Gorlato G, Zavatta M, Barbieri A. Critical illness in energy metabolism genetic disorder: rhabdomyolysis, acute kidney injury, respiratory arrest. West Indian Med J. 2013;62(8):773–5. doi:10.7727/wimj.2012.237.
Boles RG, Buck EA, Blitzer MG, Platt MS, Cowan TM, Martin SK, et al. Retrospective biochemical screening of fatty acid oxidation disorders in postmortem livers of 418 cases of sudden death in the first year of life. J Pediatr. 1998;132(6):924–33.
• Kang HM, Ahn SH, Choi P, Ko YA, Han SH, Chinga F, et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med. 2015;21(1):37–46. doi:10.1038/nm.3762. This study presents evidence linking kidney mitochondrial fatty acid oxidation to the pathogenesis of chronic kidney disease.
Adams SH, Hoppel CL, Lok KH, Zhao L, Wong SW, Minkler PE, et al. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr. 2009;139(6):1073–81. doi:10.3945/jn.108.103754.
McCoin CS, Knotts TA, Ono-Moore KD, Oort PJ, Adams SH. Long-chain acylcarnitines activate cell stress and myokine release in C2C12 myotubes: calcium-dependent and -independent effects. Am J Physiol Endocrinol Metab. 2015;308(11):E990–E1000. doi:10.1152/ajpendo.00602.2014.
Rutkowsky JM, Knotts TA, Ono-Moore KD, McCoin CS, Huang S, Schneider D, et al. Acylcarnitines activate proinflammatory signaling pathways. Am J Physiol Endocrinol Metab. 2014;306(12):E1378–87. doi:10.1152/ajpendo.00656.2013.
Procaccini C, Carbone F, Di Silvestre D, Brambilla F, De Rosa V, Galgani M, et al. The proteomic landscape of human ex vivo regulatory and conventional T cells reveals specific metabolic requirements. Immunity. 2016;44(2):406–21. doi:10.1016/j.immuni.2016.01.028.
Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186(6):3299–303. doi:10.4049/jimmunol.1003613.
Byersdorfer CA. The role of fatty acid oxidation in the metabolic reprograming of activated t-cells. Front Immunol. 2014;5:641. doi:10.3389/fimmu.2014.00641.
Shekhawat PS, Matern D, Strauss AW. Fetal fatty acid oxidation disorders, their effect on maternal health and neonatal outcome: impact of expanded newborn screening on their diagnosis and management. Pediatr Res. 2005;57(5 Pt 2):78R–86R.
Vockley J, Singh RH, Whiteman DA. Diagnosis and management of defects of mitochondrial beta-oxidation. Curr Opin Clin Nutr Metab Care. 2002;5(6):601–9. doi:10.1097/01.mco.0000038807.16540.9b.
Cecatto C, Godoy Kdos S, da Silva JC, Amaral AU, Wajner M. Disturbance of mitochondrial functions provoked by the major long-chain 3-hydroxylated fatty acids accumulating in MTP and LCHAD deficiencies in skeletal muscle. Toxicology in vitro : an international journal published in association with BIBRA. 2016;36:1–9. doi:10.1016/j.tiv.2016.06.007.
Amaral AU, Cecatto C, da Silva JC, Wajner A, Godoy Kdos S, Ribeiro RT, et al. cis-4-Decenoic and decanoic acids impair mitochondrial energy, redox and Ca(2+) homeostasis and induce mitochondrial permeability transition pore opening in rat brain and liver: possible implications for the pathogenesis of MCAD deficiency. Biochim Biophys Acta. 2016;1857(9):1363–72. doi:10.1016/j.bbabio.2016.05.007.
Hoffmann L, Seibt A, Herebian D, Spiekerkoetter U. Monounsaturated 14:1n-9 and 16:1n-9 fatty acids but not 18:1n-9 induce apoptosis and necrosis in murine HL-1 cardiomyocytes. Lipids. 2014;49(1):25–37. doi:10.1007/s11745-013-3865-4.
Akoumi A, Haffar T, Mousterji M, Kiss RS, Bousette N. Palmitate mediated diacylglycerol accumulation causes endoplasmic reticulum stress, Plin2 degradation, and cell death in H9C2 cardiomyoblasts. Exp Cell Res. 2017;354(2):85–94. doi:10.1016/j.yexcr.2017.03.032.
Krizhanovskii C, Kristinsson H, Elksnis A, Wang X, Gavali H, Bergsten P, et al. EndoC-betaH1 cells display increased sensitivity to sodium palmitate when cultured in DMEM/F12 medium. Islets. 2017:e1296995. doi:10.1080/19382014.2017.1296995.
Natarajan SK, Stringham BA, Mohr AM, Wehrkamp CJ, Lu S, Phillippi MA, et al. FoxO3 increases miR-34a to cause palmitate-induced cholangiocyte lipoapoptosis. J Lipid Res. 2017; doi:10.1194/jlr.M071357.
Kwon B, Querfurth HW. Palmitate activates mTOR/p70S6K through AMPK inhibition and hypophosphorylation of raptor in skeletal muscle cells: reversal by oleate is similar to metformin. Biochimie. 2015;118:141–50. doi:10.1016/j.biochi.2015.09.006.
Haffar T, Berube-Simard F, Bousette N. Impaired fatty acid oxidation as a cause for lipotoxicity in cardiomyocytes. Biochem Biophys Res Commun. 2015;468(1–2):73–8. doi:10.1016/j.bbrc.2015.10.162.
Nasser M, Javaheri H, Fedorowicz Z, Noorani Z. Carnitine supplementation for inborn errors of metabolism. Cochrane Database Syst Rev. 2012;2:CD006659. doi:10.1002/14651858.CD006659.pub3.
• Liepinsh E, Makrecka-Kuka M, Volska K, Kuka J, Makarova E, Antone U, et al. Long-chain acylcarnitines determine ischaemia/reperfusion-induced damage in heart mitochondria. Biochem J. 2016;473(9):1191–202. doi:10.1042/BCJ20160164. This study links acylcarnitine accumulation to infarct size in the ischemic myocardium.
Liepinsh E, Makrecka-Kuka M, Makarova E, Volska K, Svalbe B, Sevostjanovs E, et al. Decreased acylcarnitine content improves insulin sensitivity in experimental mice models of insulin resistance. Pharmacol Res. 2016;113(Pt B):788–95. doi:10.1016/j.phrs.2015.11.014.
Aguer C, McCoin CS, Knotts TA, Thrush AB, Ono-Moore K, McPherson R, et al. Acylcarnitines: potential implications for skeletal muscle insulin resistance. FASEB J. 2015;29(1):336–45. doi:10.1096/fj.14-255901.
Makrecka M, Kuka J, Volska K, Antone U, Sevostjanovs E, Cirule H, et al. Long-chain acylcarnitine content determines the pattern of energy metabolism in cardiac mitochondria. Mol Cell Biochem. 2014;395(1–2):1–10. doi:10.1007/s11010-014-2106-3.
Doi N, Tomita M, Hayashi M. Absorption enhancement effect of acylcarnitines through changes in tight junction protein in Caco-2 cell monolayers. Drug metabolism and pharmacokinetics. 2011;26(2):162–70.
Viader A, Sasaki Y, Kim S, Strickland A, Workman CS, Yang K, et al. Aberrant Schwann cell lipid metabolism linked to mitochondrial deficits leads to axon degeneration and neuropathy. Neuron. 2013;77(5):886–98. doi:10.1016/j.neuron.2013.01.012.
Goni FM, Requero MA, Alonso A. Palmitoylcarnitine, a surface-active metabolite. FEBS Lett. 1996;390(1):1–5.
Gosalakkal JA, Kamoji V. Reye syndrome and reye-like syndrome. Pediatr Neurol. 2008;39(3):198–200. doi:10.1016/j.pediatrneurol.2008.06.003.
Orlowski JP. Whatever happened to Reye’s syndrome? Did it ever really exist? Crit Care Med. 1999;27(8):1582–7.
Uppala R, Dudiak B, Beck ME, Bharathi SS, Zhang Y, Stolz DB, et al. Aspirin increases mitochondrial fatty acid oxidation. Biochem Biophys Res Commun. 2017;482(2):346–51. doi:10.1016/j.bbrc.2016.11.066.
Douglas DN, Pu CH, Lewis JT, Bhat R, Anwar-Mohamed A, Logan M, et al. Oxidative stress attenuates lipid synthesis and increases mitochondrial fatty acid oxidation in hepatoma cells infected with hepatitis C virus. J Biol Chem. 2016;291(4):1974–90. doi:10.1074/jbc.M115.674861.
Amako Y, Munakata T, Kohara M, Siddiqui A, Peers C, Harris M. Hepatitis C virus attenuates mitochondrial lipid beta-oxidation by downregulating mitochondrial trifunctional-protein expression. J Virol. 2015;89(8):4092–101. doi:10.1128/JVI.01653-14.
Feingold KR, Moser A, Patzek SM, Shigenaga JK, Grunfeld C. Infection decreases fatty acid oxidation and nuclear hormone receptors in the diaphragm. J Lipid Res. 2009;50(10):2055–63. doi:10.1194/jlr.M800655-JLR200.
Seo JY, Cresswell P. Viperin regulates cellular lipid metabolism during human cytomegalovirus infection. PLoS Pathog. 2013;9(8):e1003497. doi:10.1371/journal.ppat.1003497.
Rasmussen AL, Diamond DL, McDermott JE, Gao X, Metz TO, Matzke MM, et al. Systems virology identifies a mitochondrial fatty acid oxidation enzyme, dodecenoyl coenzyme A delta isomerase, required for hepatitis C virus replication and likely pathogenesis. J Virol. 2011;85(22):11646–54. doi:10.1128/JVI.05605-11.
Yamamoto T, Tanaka H, Emoto Y, Umehara T, Fukahori Y, Kuriu Y, et al. Carnitine palmitoyltransferase 2 gene polymorphism is a genetic risk factor for sudden unexpected death in infancy. Brain Dev. 2013; doi:10.1016/j.braindev.2013.07.011.
Kubota M, Chida J, Hoshino H, Ozawa H, Koide A, Kashii H, et al. Thermolabile CPT II variants and low blood ATP levels are closely related to severity of acute encephalopathy in Japanese children. Brain Dev. 2012;34(1):20–7. doi:10.1016/j.braindev.2010.12.012.
Deminice R, de Castro GS, Francisco LV, da Silva LE, Cardoso JF, Frajacomo FT, et al. Creatine supplementation prevents fatty liver in rats fed choline-deficient diet: a burden of one-carbon and fatty acid metabolism. J Nutr Biochem. 2015;26(4):391–7. doi:10.1016/j.jnutbio.2014.11.014.
da Silva RP, Kelly KB, Leonard KA, Jacobs RL. Creatine reduces hepatic TG accumulation in hepatocytes by stimulating fatty acid oxidation. Biochim Biophys Acta. 2014;1841(11):1639–46. doi:10.1016/j.bbalip.2014.09.001.
Greenblatt HK, Greenblatt DJ. Meldonium (mildronate): a performance-enhancing drug? Clin Pharmacol Drug Dev. 2016;5(3):167–9. doi:10.1002/cpdd.264.
Makrecka M, Svalbe B, Volska K, Sevostjanovs E, Liepins J, Grinberga S, et al. Mildronate, the inhibitor of L-carnitine transport, induces brain mitochondrial uncoupling and protects against anoxia-reoxygenation. Eur J Pharmacol. 2014;723:55–61. doi:10.1016/j.ejphar.2013.12.006.
Zhu Y, Zhang G, Zhao J, Li D, Yan X, Liu J, et al. Efficacy and safety of mildronate for acute ischemic stroke: a randomized, double-blind, active-controlled phase II multicenter trial. Clinical drug investigation. 2013;33(10):755–60. doi:10.1007/s40261-013-0121-x.
Vilskersts R, Liepinsh E, Kuka J, Cirule H, Veveris M, Kalvinsh I, et al. Myocardial infarct size-limiting and anti-arrhythmic effects of mildronate orotate in the rat heart. Cardiovascular drugs and therapy / sponsored by the International Society of Cardiovascular Pharmacotherapy. 2009;23(4):281–8. doi:10.1007/s10557-009-6179-2.
Jaudzems K, Kuka J, Gutsaits A, Zinovjevs K, Kalvinsh I, Liepinsh E, et al. Inhibition of carnitine acetyltransferase by mildronate, a regulator of energy metabolism. Journal of enzyme inhibition and medicinal chemistry. 2009;24(6):1269–75. doi:10.3109/14756360902829527.
Liepinsh E, Konrade I, Skapare E, Pugovics O, Grinberga S, Kuka J, et al. Mildronate treatment alters gamma-butyrobetaine and l-carnitine concentrations in healthy volunteers. J Pharm Pharmacol. 2011;63(9):1195–201. doi:10.1111/j.2042-7158.2011.01325.x.
Park HS, Jang JE, Ko MS, Woo SH, Kim BJ, Kim HS, et al. Statins increase mitochondrial and peroxisomal fatty acid oxidation in the liver and prevent non-alcoholic steatohepatitis in mice. Diabetes Metab J. 2016;40(5):376–85. doi:10.4093/dmj.2016.40.5.376.
Zhang J, Zhang W, Zou D, Chen G, Wan T, Zhang M, et al. Cloning and functional characterization of ACAD-9, a novel member of human acyl-CoA dehydrogenase family. Biochem Biophys Res Commun. 2002;297(4):1033–42.
Ensenauer R, He M, Willard JM, Goetzman ES, Corydon TJ, Vandahl BB, et al. Human acyl-CoA dehydrogenase-9 plays a novel role in the mitochondrial beta-oxidation of unsaturated fatty acids. J Biol Chem. 2005;280(37):32309–16. doi:10.1074/jbc.M504460200.
He M, Rutledge SL, Kelly DR, Palmer CA, Murdoch G, Majumder N, et al. A new genetic disorder in mitochondrial fatty acid beta-oxidation: ACAD9 deficiency. Am J Hum Genet. 2007;81(1):87–103. doi:10.1086/519219.
• Nouws J, Nijtmans L, Houten SM, van den Brand M, Huynen M, Venselaar H, et al. Acyl-CoA dehydrogenase 9 is required for the biogenesis of oxidative phosphorylation complex I. Cell Metab. 2010;12(3):283–94. doi:10.1016/j.cmet.2010.08.002. This study discovered a novel role for ACAD9 in regulating the assembly of electron transport chain complex I.
Dewulf JP, Barrea C, Vincent MF, De Laet C, Van Coster R, Seneca S, et al. Evidence of a wide spectrum of cardiac involvement due to ACAD9 mutations: report on nine patients. Mol Genet Metab. 2016;118(3):185–9. doi:10.1016/j.ymgme.2016.05.005.
Schiff M, Haberberger B, Xia C, Mohsen A-W, Goetzman ES, Wang Y, et al. Complex I assembly function and fatty acid oxidation enzyme activity of ACAD9 both contribute to disease severity in ACAD9 deficiency. Hum Mol Genet. 2015;24(11):3238–47. doi:10.1093/hmg/ddv074.
Lagoutte-Renosi J, Segalas-Milazzo I, Crahes M, Renosi F, Menu-Bouaouiche L, Torre S, et al. Lethal neonatal progression of fetal cardiomegaly associated to ACAD9 deficiency. JIMD reports. 2015; doi:10.1007/8904_2015_499.
Haack TB, Danhauser K, Haberberger B, Hoser J, Strecker V, Boehm D, et al. Exome sequencing identifies ACAD9 mutations as a cause of complex I deficiency. Nat Genet. 2010;42(12):1131–4. doi:10.1038/ng.706.
• Peters H, Buck N, Wanders R, Ruiter J, Waterham H, Koster J, et al. ECHS1 mutations in Leigh disease: a new inborn error of metabolism affecting valine metabolism. Brain. 2014;137(Pt 11):2903–8. doi:10.1093/brain/awu216. This study describes the first patients with ECHS1 deficiency.
Haack TB, Jackson CB, Murayama K, Kremer LS, Schaller A, Kotzaeridou U, et al. Deficiency of ECHS1 causes mitochondrial encephalopathy with cardiac involvement. Ann Clin Transl Neurol. 2015;2(5):492–509. doi:10.1002/acn3.189.
He M, Pei Z, Mohsen AW, Watkins P, Murdoch G, Van Veldhoven PP, et al. Identification and characterization of new long chain acyl-CoA dehydrogenases. Mol Genet Metab. 2011;102(4):418–29. doi:10.1016/j.ymgme.2010.12.005.
Bloom K, Mohsen AW, Karunanidhi A, El Demellawy D, Reyes-Mugica M, Wang Y, et al. Investigating the link of ACAD10 deficiency to type 2 diabetes mellitus. J Inherit Metab Dis. 2017; doi:10.1007/s10545-017-0013-y.
Bian L, Hanson RL, Muller YL, Ma L, Investigators M, Kobes S, et al. Variants in ACAD10 are associated with type 2 diabetes, insulin resistance and lipid oxidation in Pima Indians. Diabetologia. 2010;53(7):1349–53. doi:10.1007/s00125-010-1695-y.
Wu L, Zhou B, Oshiro-Rapley N, Li M, Paulo JA, Webster CM, et al. An ancient, unified mechanism for metformin growth inhibition in C. elegans and cancer. Cell. 2016;167(7):1705–18 e13. doi:10.1016/j.cell.2016.11.055.
van Eerd DC, Brusse IA, Adriaens VF, Mankowski RT, Praet SF, Michels M, et al. Management of an LCHADD patient during pregnancy and high intensity exercise. JIMD reports. 2017;32:95–100. doi:10.1007/8904_2016_561.
Behrend AM, Harding CO, Shoemaker JD, Matern D, Sahn DJ, Elliot DL, et al. Substrate oxidation and cardiac performance during exercise in disorders of long chain fatty acid oxidation. Mol Genet Metab. 2012;105(1):110–5. doi:10.1016/j.ymgme.2011.09.030.
Gillingham MB, Scott B, Elliott D, Harding CO. Metabolic control during exercise with and without medium-chain triglycerides (MCT) in children with long-chain 3-hydroxy acyl-CoA dehydrogenase (LCHAD) or trifunctional protein (TFP) deficiency. Mol Genet Metab. 2006;89(1–2):58–63. doi:10.1016/j.ymgme.2006.06.004.
Li H, Fukuda S, Hasegawa Y, Kobayashi H, Purevsuren J, Mushimoto Y, et al. Effect of heat stress and bezafibrate on mitochondrial beta-oxidation: comparison between cultured cells from normal and mitochondrial fatty acid oxidation disorder children using in vitro probe acylcarnitine profiling assay. Brain Dev. 2010;32(5):362–70. doi:10.1016/j.braindev.2009.06.001.
Orngreen MC, Vissing J, Laforet P. No effect of bezafibrate in patients with CPTII and VLCAD deficiencies. J Inherit Metab Dis. 2015;38(2):373–4. doi:10.1007/s10545-014-9779-3.
Orngreen MC, Madsen KL, Preisler N, Andersen G, Vissing J, Laforet P. Bezafibrate in skeletal muscle fatty acid oxidation disorders: a randomized clinical trial. Neurology. 2014;82(7):607–13. doi:10.1212/WNL.0000000000000118.
Roe CR, Sweetman L, Roe DS, David F, Brunengraber H. Treatment of cardiomyopathy and rhabdomyolysis in long-chain fat oxidation disorders using an anaplerotic odd-chain triglyceride. J Clin Invest. 2002;110(2):259–69. doi:10.1172/JCI15311.
Vockley J, Burton B, Berry GT, Longo N, Phillips J, Sanchez-Valle A, et al. UX007 for the treatment of long chain-fatty acid oxidation disorders: safety and efficacy in children and adults following 24weeks of treatment. Mol Genet Metab. 2017;120(4):370–7. doi:10.1016/j.ymgme.2017.02.005.
Vockley J, Marsden D, McCracken E, DeWard S, Barone A, Hsu K, et al. Long-term major clinical outcomes in patients with long chain fatty acid oxidation disorders before and after transition to triheptanoin treatment—a retrospective chart review. Mol Genet Metab. 2015;116(1–2):53–60. doi:10.1016/j.ymgme.2015.06.006.
Palir N, Ruiter JP, Wanders RJ, Houtkooper RH. Identification of enzymes involved in oxidation of phenylbutyrate. J Lipid Res. 2017; doi:10.1194/jlr.M075317.
Kormanik K, Kang H, Cuebas D, Vockley J, Mohsen AW. Evidence for involvement of medium chain acyl-CoA dehydrogenase in the metabolism of phenylbutyrate. Mol Genet Metab. 2012;107(4):684–9. doi:10.1016/j.ymgme.2012.10.009.
Cullis PR, Hope MJ. Lipid nanoparticle systems for enabling gene therapies. Molecular therapy : the journal of the American Society of Gene Therapy. 2017; doi:10.1016/j.ymthe.2017.03.013.
Keeler AM, ElMallah MK, Flotte TR. Gene therapy 2017: progress and future directions. Clin Transl Sci. 2017; doi:10.1111/cts.12466.
Zhang Y, Bharathi SS, Rardin MJ, Uppala R, Verdin E, Gibson BW, et al. SIRT3 and SIRT5 regulate the enzyme activity and cardiolipin binding of very long-chain acyl-CoA dehydrogenase. PLoS One. 2015;10(3):e0122297. doi:10.1371/journal.pone.0122297.
Rardin MJ, He W, Nishida Y, Newman JC, Carrico C, Danielson SR, et al. SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks. Cell Metab. 2013;18(6):920–33. doi:10.1016/j.cmet.2013.11.013.
Bharathi SS, Zhang Y, Mohsen AW, Uppala R, Balasubramani M, Schreiber E, et al. Sirtuin 3 (SIRT3) protein regulates long-chain acyl-CoA dehydrogenase by deacetylating conserved lysines near the active site. J Biol Chem. 2013;288(47):33837–47. doi:10.1074/jbc.M113.510354.
Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464(7285):121–U37. doi:10.1038/nature08778.
Wang Y, Mohsen AW, Mihalik SJ, Goetzman ES, Vockley J. Evidence for physical association of mitochondrial fatty acid oxidation and oxidative phosphorylation complexes. J Biol Chem. 2010;285(39):29834–41. doi:10.1074/jbc.M110.139493.
Doulias PT, Tenopoulou M, Greene JL, Raju K, Ischiropoulos H. Nitric oxide regulates mitochondrial fatty acid metabolism through reversible protein S-nitrosylation. Sci Signal. 2013;6(256):rs1. doi:10.1126/scisignal.2003252.
• Tenopoulou M, Chen J, Bastin J, Bennett MJ, Ischiropoulos H, Doulias PT. Strategies for correcting very long chain acyl-CoA dehydrogenase deficiency. J Biol Chem. 2015;290(16):10486–94. doi:10.1074/jbc.M114.635102. This study demonstrates manipulation of a post-translational modification as a potential new therapeutic strategy for VLCAD deficiency.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Clinical Genetics
Rights and permissions
About this article
Cite this article
Goetzman, E.S. Advances in the Understanding and Treatment of Mitochondrial Fatty Acid Oxidation Disorders. Curr Genet Med Rep 5, 132–142 (2017). https://doi.org/10.1007/s40142-017-0125-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40142-017-0125-6