Abstract
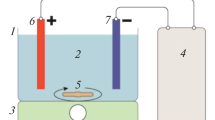
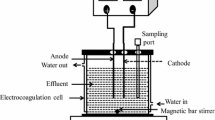
The present work reports treatment of synthetic phenolic wastewater by electrocoagulation process. Aluminum flat sheets were utilized as electrodes. Central composite design combined with response surface methodology has been applied for optimizing the process parameters. The interaction effects of phenol concentration, electrode distance, pH, voltage, and electrolysis time (ET) were analyzed and correlated to assess the efficiency of phenol removal as process response. The ANOVA outcomes declared that the initial phenol concentration (relevant coefficient = −3.44) and ET (relevant coefficient = 1.42), respectively, are the most and the least effective parameters on the efficiency of phenol removal. Furthermore, optimal factors were obtained as follows: influent phenol concentration = 14.23 mg/L, electrode distance = 2.20 cm, pH = 6.37, voltage = 16.46 V, and electrolysis time = 44.66 min, in which the percentage of phenol removal at this condition was about 90.6%.







Similar content being viewed by others
Abbreviations
- Adj. R 2 :
-
Adjusted R2
- ANOVA:
-
Analysis of variance
- AP:
-
Adequate precision
- CCD:
-
Central composite design
- COD:
-
Chemical oxygen demand
- CV:
-
Coefficients of variation
- EC:
-
Electrocoagulation
- ED:
-
Electrode distance
- ET:
-
Electrolysis time
- R 2 :
-
Determination coefficient
- RSM:
-
Response surface methodology
- SD:
-
Standard deviation
References
Abdelwahab O, Amin N, El-Ashtoukhy EZ (2009) Electrochemical removal of phenol from oil refinery wastewater. J Hazard Mater 163:711–716
Adhoum N, Monser L (2004) Decolourization and removal of phenolic compounds from olive mill wastewater by electrocoagulation. Chem Eng Process 43:1281–1287
Akintunde AM, Ajala SO, Betiku E (2015) Optimization of Bauhinia monandra seed oil extraction via artificial neural network and response surface methodology: a potential biofuel candidate. Ind Crops Prod 67:387–394
Bas D, Boyaci IH (2007) Modeling and optimization II: comparison of estimation capabilities of response surface methodology with artificial neural networks in a biochemical reaction. J Food Eng 78:846–854
Bódalo A, Gómez E, Hidalgo A, Gómez M, Murcia M, López I (2009) Nanofiltration membranes to reduce phenol concentration in wastewater. Desalination 245:680–686
Box GE, Hunter WG, Hunter JS (1978) Statistics for experimenters: an introduction to design, data analysis, and model building, vol 1. JSTOR, New York
Danmaliki GI, Saleh TA (2017) Effects of bimetallic Ce/Fe nanoparticles on the desulfurization of thiophenes using activated carbon. Chem Eng J 307:914–927
Danmaliki GI, Saleh TA, Shamsuddeen AA (2017) Response surface methodology optimization of adsorptive desulfurization on nickel/activated carbon. Chem Eng J 313:993–1003
Deng Y (2007) Physical and oxidative removal of organics during Fenton treatment of mature municipal landfill leachate. J Hazard Mater 146:334–340
El-Ashtoukhy EZ, Amin N (2010) Removal of acid green dye 50 from wastewater by anodic oxidation and electrocoagulation—a comparative study. J Hazard Mater 179:113–119
El-Ashtoukhy E, El-Taweel Y, Abdelwahab O, Nassef E (2013) Treatment of petrochemical wastewater containing phenolic compounds by electrocoagulation using a fixed bed electrochemical reactor. Int J Electrochem Sci 8:1534–1550
El-Naas MH, Al-Zuhair S, Alhaija MA (2010) Removal of phenol from petroleum refinery wastewater through adsorption on date-pit activated carbon. Chem Eng J 162:997–1005
Emamjomeh MM, Sivakumar M (2009) Review of pollutants removed by electrocoagulation and electrocoagulation/flotation processes. J Environ Manage 90:1663–1679
Ferri F, Bertin L, Scoma A, Marchetti L, Fava F (2011) Recovery of low molecular weight phenols through solid-phase extraction. Chem Eng J 166:994–1001
Gupta VK, Ali I, Saleh TA, Nayak A, Agarwal S (2012) Chemical treatment technologies for waste-water recycling—an overview. Rsc Adv 2:6380–6388
Kadlec RH, Zmarthie LA (2010) Wetland treatment of leachate from a closed landfill. Ecol Eng 36:946–957
Katal R, Pahlavanzadeh H (2011) Influence of different combinations of aluminum and iron electrode on electrocoagulation efficiency: application to the treatment of paper mill wastewater. Desalination 265:199–205
Kennedy LJ, Vijaya JJ, Kayalvizhi K, Sekaran G (2007) Adsorption of phenol from aqueous solutions using mesoporous carbon prepared by two-stage process. Chem Eng J 132:279–287
Kobya M, Demirbas E, Can O, Bayramoglu M (2006) Treatment of levafix orange textile dye solution by electrocoagulation. J Hazard Mater 132:183–188
Körbahti BK, Tanyolaç A (2008) Electrochemical treatment of simulated textile wastewater with industrial components and Levafix Blue CA reactive dye: optimization through response surface methodology. J Hazard Mater 151:422–431
Lazo-Cannata JC, Nieto-Márquez A, Jacoby A, Paredes-Doig AL, Romero A, Sun-Kou MR, Valverde JL (2011) Adsorption of phenol and nitrophenols by carbon nanospheres: effect of pH and ionic strength. Sep Purif Technol 80:217–224
Lomax RG, Hahs-Vaughn DL (2013) Statistical concepts: a second course. Routledge, London
Mahvi AH, Maleki A, Alimohamadi M, Ghasri A (2007) Photo-oxidation of phenol in aqueous solution: toxicity of intermediates. Korean J Chem Eng 24:79–82
Maleki A, Mahvi A, Mesdaghinia A, Naddafi K (2007) Degradation and toxicity reduction of phenol by ultrasound waves. Bull Chem Soc Ethiop 21:33–38
Mason RL, Gunst RF, Hess JL (2003) Statistical design and analysis of experiments: with applications to engineering and science, vol 474. Wiley, New York
Mohora E, Rončević S, Dalmacija B, Agbaba J, Watson M, Karlović E, Dalmacija M (2012) Removal of natural organic matter and arsenic from water by electrocoagulation/flotation continuous flow reactor. J Hazard Mater 235:257–264
Moussavi G, Barikbin B, Mahmoudi M (2010) The removal of high concentrations of phenol from saline wastewater using aerobic granular SBR. Chem Eng J 158:498–504
Myers RH, Montgomery DC, Anderson-Cook CM (2016) Response surface methodology: process and product optimization using designed experiments. Wiley, New York
Nasrullah M, Singh L, Wahida ZA (2012) Treatment of sewage by electrocoagulation and the effect of high current density. Energy Environ Eng J 1(1):27–31
Parga JR et al (2005) Arsenic removal via electrocoagulation from heavy metal contaminated groundwater in La Comarca Lagunera Mexico. J Hazard Mater 124:247–254
Pouet M-F, Grasmick A (1995) Urban wastewater treatment by electrocoagulation and flotation. Water Sci Technol 31:275–283
Rajendran S, Khan MM, Gracia F, Qin J, Gupta VK, Arumainathan S (2016) Ce3+-ion-induced visible-light photocatalytic degradation and electrochemical activity of ZnO/CeO2 nanocomposite. Sci Rep 6:31641
Rijsberman FR (2006) Water scarcity: Fact or fiction? Agric Water Manage 80:5–22
Saleh TA (2015) Isotherm, kinetic, and thermodynamic studies on Hg(II) adsorption from aqueous solution by silica-multiwall carbon nanotubes. Environ Sci Pollut Res 22:16721–16731
Saleh TA (2016) Nanocomposite of carbon nanotubes/silica nanoparticles and their use for adsorption of Pb(II): from surface properties to sorption mechanism. Desalin Water Treat 57:10730–10744
Saleh TA, Sarı A, Tuzen M (2017) Effective adsorption of antimony (III) from aqueous solutions by polyamide–graphene composite as a novel adsorbent. Chem Eng J 307:230–238
Saravanan R, Karthikeyan N, Gupta V, Thirumal E, Thangadurai P, Narayanan V, Stephen A (2013a) ZnO/Ag nanocomposite: an efficient catalyst for degradation studies of textile effluents under visible light. Mater Sci Eng C 33:2235–2244
Saravanan R, Karthikeyan S, Gupta V, Sekaran G, Narayanan V, Stephen A (2013b) Enhanced photocatalytic activity of ZnO/CuO nanocomposite for the degradation of textile dye on visible light illumination. Mater Sci Eng C 33:91–98
Saravanan R, Gupta V, Mosquera E, Gracia F (2014) Preparation and characterization of V2O5/ZnO nanocomposite system for photocatalytic application. J Mol Liq 198:409–412
Saravanan R, Gracia F, Khan MM, Poornima V, Gupta VK, Narayanan V, Stephen A (2015) ZnO/CdO nanocomposites for textile effluent degradation and electrochemical detection. J Mol Liq 209:374–380
Shailubhai K (1986) Treatment of petroleum industry oil sludge in soil. Trends Biotechnol 4:202–206
Tsilogeorgis J, Zouboulis A, Samaras P, Zamboulis D (2008) Application of a membrane sequencing batch reactor for landfill leachate treatment. Desalination 221:483–493
Wang F et al (2014) Optimization of methazolamide-loaded solid lipid nanoparticles for ophthalmic delivery using Box–Behnken design. J Liposome Res 24:171–181
Zhu J, Zhao H, Ni J (2007) Fluoride distribution in electrocoagulation defluoridation process. Sep Purif Technol 56:184–191
Acknowledgements
The authors would like to thank the water treatment plant located in Nanaleh County of Sanandaj, Iran, and also Miss Haleh Nourizadeh and Mr. Kamel Rouzrokh for their kind cooperation. Furthermore, the authors are grateful to State-Ease, Minneapolis, MN, USA, for the provision of the Design-Expert package.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: V.K. Gupta.
Rights and permissions
About this article
Cite this article
Karami, T., Elyasi, S. & Amani, T. Modeling and optimizing of electrocoagulation process in treating phenolic wastewater by response surface methodology: precise evaluation of significant variables. Int. J. Environ. Sci. Technol. 15, 2389–2398 (2018). https://doi.org/10.1007/s13762-017-1539-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-017-1539-0