Abstract
Management and conservation of wild pollinators in forests of the Chaco region of South America is of great interest nowadays as they are pollen vectors that improve the reproductive success of many forest species which are at risk due mainly to forest patch isolation and degradation by human activities. The assessment of the diet of the stingless bee Melipona orbignyi was carried out by comparing different calculations from palynological data being the index of relative importance (IRI) the most complete, objective, and here recommended. This index showed that the order of the four most important pollen resources was Prosopis (IRI = 3354), Albizia inundata (1999), Cynophalla retusa (921), and Solanum type 2 (693), and the value of importance of species (SI) calculated for honey resources showed that they were type Maytenus (SI = 20.79), Prosopis (18.73), Cynophalla retusa (6.72), and Ziziphus mistol (3.32). Three flower types predominated in pollen and nectar provisions: brush flowers, solanoid flowers with poricidal anthers, and generalized small pale flowers, being the former two associated to buzzing. The abundance of the nectarless Solanum in honey samples is discussed. Good forest management practices for pollinator conservation native to the Chaco forest should include the keeping of undisturbed forest patches for nesting and foraging while the surrounding forest resources are managed in a sustainable way. Furthermore, because some threatened plants important for the M. orbignyi diet require gene flow to maintain healthy populations, short distances among the forest patches are needed, a fact that also helps to avoid genetic drift in Melipona colonies. The rational rearing of Melipona orbignyi would be a good practice to be implemented to promote pollination of threatened tree and shrub individuals isolated by forest fragmentation.
Similar content being viewed by others
1 Introduction
The knowledge of the diet of wild pollen vectors provides interesting information on plant-pollinator interaction and potential pollination in diverse communities. Among pollinators, stingless bees are a special group as their perennial colonies of many individuals can be reared inside boxes or trunks in a rational way for many years and so increasing the pollination of the surrounding native plants (Venturieri et al. 2003; Cortopassi-Laurino et al. 2006; Roubik 2006). Among meliponines, Melipona species are especially important in forest conservation as they are capable of cross-pollination within 2 to 2.4-km radius (Roubik and Aluja 1983; Araújo et al. 2004). On the other hand, the remaining meliponines concentrate their foraging within short distances from their nests, typically on crowns of mass-flowering trees with small generalized flowers (Wilms et al. 1997; Ramalho 2004; Monteiro and Ramalho 2010). Nowadays, reproduction of most Chaquenian forest species is being threatened by degrading human activities and fragmentation as patches are many times small and are far apart (Aizen and Feinsinger 1994a, 1994b; Lin and Bernardello 1999; Zak et al. 2004; Ferreyra et al. 2007; Vesprini et al. 2011; Bessega et al. 2012). This is also true for many forest around the world (Brown and Albrecht 2001; Brosi et al. 2008; Brosi 2009; Ferreira et al. 2015). Melipona orbignyi (Guérin) belongs to Melipona group favosa (Camargo and Pedro 2013), which includes many circum-Amazonian species from Argentina (25° S) to Panamá (9° N) (Nates-Parra and Roubik 1990). According to the interpretation of Nates-Parra and Roubik (1990), M. orbignyi is a polytypic species (these authors merged it with Melipona phenax Cockerell and Melipona lunulata Friese); therefore, M. orbignyi ranges from Argentina to Panamá. Schwarz (1932) had noticed that coloration patterns are variable among these taxa including Melipona baeri Vachal. Thus, the polytypic M. orbignyi reaches the southernmost distributional limit in the Chaco xerophytic forest of South America (25° S).
Diet assessment can be effectively achieved by means of pollen analysis of provisions stored in stingless bee nests (Ramalho et al. 1989, 2007; Vossler et al. 2010; Vossler 2015a, 2015b, 2018), providing diverse information helpful for conservation and pollination purposes (Vossler et al. 2018). The relative abundance of pollen types and its frequency in samples have been the two calculations most used in bee diet studies. However, the order of importance of plants in the bee diet has been selected arbitrarily by many authors; in many instances, they were chosen for being the most abundant or the ones with the highest frequency, or a combination of both (Lieux 1981; Obregón and Nates-Parra 2014), although some studies included a more accurate determination by combining them in a formula (Nates-Parra et al. 2013), or the most abundant and the volume biomass (Vossler et al. 2010) or the three components together (Dalmazzo and Vossler 2015). Pollen volume biomass is important in the study of bee diets, as it can greatly vary among the plants foraged. Another method includes the ranking of the total harvested pollen volume (Villanueva-Gutiérrez and Roubik 2004). Plant resources foraged by bees and stored in nests have also successfully been estimated by indices of diversity and evenness (Kleinert-Giovannini and Imperatriz-Fonseca 1987; Cortopassi-Laurino and Ramalho 1988; Kajobe 2006; Cortopassi-Laurino et al. 2009; Vossler et al. 2010; Hilgert-Moreira et al. 2013; Ferreira and Absy 2015) and multivariate methods (Ramalho et al. 2007; Rech and Absy 2011; Vossler 2013, submitted; Vossler et al. 2014).
Stingless bees (tribe Meliponini) are considered as a broadly polylectic bee group (Cane and Sipes 2006); however, different degrees of polylecty were detected for different species and genera according to the spatial and temporal analysis of the sampling performed (Vossler 2018). Only Melipona species are able to vibrate flowers to legitimately collect large amounts of pollen grains from poricidal anthers usually from Solanum (Solanaceae), Senna (Fabaceae, Papilionoideae, Cassiinae) and Melastomataceae (Ramalho et al. 1989; Marques-Souza 1996; Nunes-Silva et al. 2010; Ferreira and Absy 2015). Convergent in many lineages of bees, buzzing allowed to Melipona the usage of exclusive floral resources and in consequence the avoidance of competition with no buzzing bees. Ramalho et al. (1989) argued that flowers specialized on tropical Melipona compared with the remaining stingless bees were bisexual, of radial symmetry, white to purple colored and some with poricidal anthers. At family level, it is interesting to mention that two of the main pollen resources usually recorded for Melipona in the Neotropics (Myrtaceae and Melastomataceae) are absent in the dry forest of the Chaco region, although the remaining ones (Solanaceae, Fabaceae (the three subfamilies), Arecaceae, Anacardiaceae and Sapindaceae) (Ramalho et al. 1989, 2007; Marques-Souza et al. 1995) are abundant. It is also possible that new pollen families as well as pollination syndromes with specific flower morphology were found in this subtropical dry forest of South America and that they were highly represented in the diet of Melipona orbignyi (Guérin).
The aims of the present study were to provide information on the botanical origin of pollen and honey provisions, assess the most important resources in the diet by comparing different calculations and identify flower syndromes associated with Melipona orbignyi in its southernmost distributional limit. Furthermore, recommendations on management and conservation of native bee pollinators in the Chaco xerophytic forest of South America were provided.
2 Materials and methods
2.1 Study area: the Chaco dry forest in South America
The Chaco region is a large sedimentary plain of about 1000,000 km2, extending both north and south of the Tropic of Capricorn over northern Argentina, western Paraguay, eastern Bolivia, and part of southeastern Brazil. The original vegetation consisted of a mosaic formed by xerophytic forests, gallery forests and soil-determined or fire-generated savannas (Adámoli et al. 1990). The present survey was carried out in a continuous dry forest locally named ‘palosantal’ due to a dominant zygophyllaceous tree, the ‘palo santo’ (Bulnesia sarmientoi). The climate at the study area is strongly seasonal with very hot summer (December to March) and low temperatures and frost during winter (July to September) (Prado 1993); there is a great yearly variation in rainfall, with a marked dry season in winter-spring and a rainy season from October to April (Papadakis 1973).
2.2 Pollen analysis of pollen and honey provisions and plant and bee references
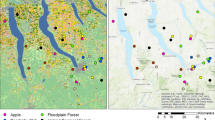
A total of eight honey and nine pollen provision samples were palynologically analyzed from nine nests sampled in a xerophilous forest at two sites in the Argentinean Chaco: El Espinillo (25° 24′ S, 60° 27′ W) and El Sauzalito (24° 24′ S, 61° 40′ W) (Figure 1a, b; Table I). Dense clusters of honey and pollen pots were taken from colonies between February 2006 and February 2009. Honey from 1 to 70 and pollen provision from 1 to 20 pots per nest (and pollen from approximately 550 brood cells in nest 1) were studied. Closed pots were preferred for sampling honey, as they contained pure honey. However, honey was also sampled when only open pots were available (these honey samples were probably contaminated during their sampling with pollen grains from pot pollen, and the interpretation on nectar resources was cautiously done). The honey content from different pots from each nest was mixed and this analyzed per each nest. In the case of pollen provisions, one slide per pot was obtained from all pots sampled (see Table I), and their abundance values were averaged, and therefore, one value per nest was presented (nest pollen analysis). In the case of broken pollen pots, their content was mixed, and one slide was obtained per nest (Table I). Moist pollen from broken pollen pots was also considered as probably contaminated from honey, but the abundance of pollen grains in honey is minimal in comparison with the one present in pollen pots. Honey samples were dissolved in distilled water at 80–90 °C and immediately stirred with a magnetic stirrer for 10–15 min. The same steps were performed for pollen provision samples, but they were hydrated for up to 24 h to complete individual pollen load dissolution. A representative mixture of 5–10 ml was obtained and centrifuged at 472×g for 5 min. The processing of honey included standard acetolysis (Erdtman 1960) followed by counting of 500 pollen grains per slide. Pollen provisions were acetolyzed, and a total of 300–500 pollen grains were counted per sample, as in most slides, no new types were identified when 300 grains were counted. Pollen identification was carried out by comparing pollen provision slides with the pollen reference of plants grown in the sites sampled, using a light microscope Nikon Eclipse E200. Pollen-type nomenclature follows the recommendations of Joosten and De Klerk (2002) and De Klerk and Joosten (2007) (see ESM 1). Pollen types were photographed using a scanning electron microscope PHILIPS XL30 (Figure 1c, e, g). The reference pollen collection was made from flower buds of plant species collected in various localities from the Chaco province of Argentina (Juan José Castelli, Villa Río Bermejito, Miraflores, El Espinillo and El Sauzalito). These plant specimens were pressed, dried, identified by the author, and deposited in the Herbarium of the Museo de La Plata (LP), the Herbarium of Museo Argentino de Ciencias Naturales ‘Bernardino Rivadavia’ (BA), Buenos Aires, and the Herbarium Lorentz (DTE) of Diamante, Entre Ríos, Argentina. Flower types were photographed (Figures 1d and 2). Bee specimens were collected from nests, identified by Arturo Roig-Alsina, and deposited in the Entomology Collection of the Museo Argentino de Ciencias Naturales ‘Bernardino Rivadavia’, Buenos Aires, Argentina.
a Location of sampling sites in the Chaco region of South America [El Espinillo 25° 24′ S, 60° 27′ W (site 1) and El Sauzalito 24° 24′ S, 61° 40′ W (site 2)]. b The ‘palosantal’ dry forest during October; many ‘palo santo’ (Bulnesia sarmientoi) trees at the highest stratum can be seen. c, e Scanning photomicrograph of pollen grains found in pollen provisions of Melipona orbignyi showing a great variety of pollen volumes: tetrad of Mimosa (1), polyad of Albizia inundata (2), Prosopis (3), Cynophalla retusa (4), type Senegalia praecox (5) and type Vachellia aroma (6) (bar = 20 μm (c), 100 μm (e)). d Pollen collection by buzzing on the ‘solanoid type’ flower of Solanum aridum (Solanaceae). Pollen loads can be seen on the corbiculae. f Nest entrance of M. orbignyi in a living tree trunk of Ziziphus mistol. g Scanning photomicrograph of the pollen grain of Solanum (bar = 10 μm).
2.3 Calculations performed to assess bee diet (Figure 3)
2.3.1 Absolute volume of pollen grains
To estimate the absolute volume of pollen used by bees, fresh grains from reference plants (and from pollen provision samples when necessary) were dyed using fuchsine (but not acetolyzed), mounted in slides using a glycerine-gelatin mixture and measured using a light microscope Nikon Eclipse E200 at ×1000 magnification and photographed using a scanning electron microscope PHILIPS XL30 (Figure 1c, e, g). The absolute volume (mean ± SD) of each pollen type/pollen species was estimated by means of the measurement of axes p and e for ellipsoidal and spherical shapes (equatorial view) and h and s for prismatic ones (two views) of 9 to 15 pollen grains per plant species, following Vossler (2015a). Only the cytoplasm was measured (excluding the exine) as it provides almost the total amount of the digestible biomass (Ueira-Vieira et al. 2013). The allocation in pollen volume categories (Vossler 2015a) was also done.
2.3.2 Types of pollen counts
The direct pollen count (i.e., the relative abundance of pollen types) was applied for honey and pollen samples and expressed as percentage (Figure 3; Table II). In contrast, the relative volume was only performed for pollen samples and calculated multiplying the mean absolute volume for each pollen type/pollen species by its absolute number of grains counted per sample, divided by the sum of all these values and express as percentage (Figure 3; Table II). The chi-squared (χ2) test (α = 0.05) was used for comparison of percentage values of the population of pollen types of each kind of count per nest pollen sample (Table II). Pollen types occurring in more than 5% were considered as the abundant resources (versus minor, those ≤ 5%) following Vossler (2018).
2.3.3 Indices of diversity of Shannon-Wiener (H′) and evenness (J′)
To calculate the diversity (H′) and evenness (J′) of the pollen types, the version 1.81 of PAST statistical package (Hammer et al. 2008) was used. The diversity index (H′) (Hutcheson 1970) was used to estimate the trophic niche size of M. orbignyi, which is expressed as H′ = − Σ pi ln (pi), where pi is the proportion of the pollen type i. Evenness index (J′) was calculated according to Pielou (1977): J′ = H′ / S, where S = ln P, and P is the number of pollen types per sample. Both indices were calculated for pollen and honey provisions from the direct pollen count and relative volume (Figure 3). To compare the H′ index between nests, the t test was applied using natural logarithm with α = 0.05.
2.3.4 Value of importance of species
To estimate the order of importance of the honey resources, the value of importance of species (SI) was used, being SI j = mean relative abundance j × number of samples j / total number of samples, where j is each pollen type (Nates-Parra et al. 2013). The SI was also used for pollen resources comparing this result with that provided by index of relative importance (IRI) (Table III).
2.3.5 Index of relative importance of pollen hosts in the diet
For ranking the relative importance of pollen types that composed the pollen diet of this stingless bee, the IRI was estimated using the formula: IRI = (N + V)F, where N = numerical percentage, V = volumetric percentage, and F = frequency of occurrence percentage (Figure 3; Table IV) (Pinkas et al. 1971; Hart et al. 2002). The IRI was here used for the first time in stingless bees and for the second time in bees (in the halictid Augochlora amphitrite by Dalmazzo and Vossler (2015)). As pollen grain volume is irrelevant in the nectar diet, IRI is only calculated from pollen resources. The IRI takes into account not only the relative abundance of each pollen type but also its relative volume and frequency of occurrence in samples; the higher the combination of values of these three components, the higher the IRI value. The numerical percentage (N value) was calculated by counting the number of grains (or another unit of dispersion such as polyad) per pollen type, and then, they were added across the nine nests. This value was divided by the total sum of all these values and multiplied by 100 (Table IV). To calculate the volumetric percentage (V value), the mean absolute volume of each pollen type was divided by the sum of mean absolute volume of all types (23 out of the 25 types foraged were calculated) and then multiplied by 100 (Table IV). And, frequency of occurrence percentage (F value) is the percentage of samples containing each pollen type. As example, the pollen type Prosopis was found in eight out of the nine nests; therefore, 8 was divided by 9 and multiplied by 100 obtaining thus the F value (88.89%). Details of calculation of IRI can be found in Dalmazzo and Vossler (2015). To calculate the IRI value of the type Maytenus, absolute volume from reference pollen grains from Maytenus vitis-idaea and Moya spinosa was averaged. IRI was not calculated for types Unidentified 5 and Unidentified 6 as their absolute volume could not be estimated because only a few grains were available. As only a few grains of Solanum type 1 were found in samples, volume value of Solanum type 2 of similar size was used.
3 Results
3.1 Botanical origin of pollen and honey provisions
From nine nests analyzed, a total of 30 pollen types were found in both provision types. A total of 25 pollen types from 15 families were present in pollen provisions; 10 types of those from 7 families were abundantly represented (˃ 5%). In decreasing order of their maximum value (from direct pollen count), they were type Schinopsis (Anacardiaceae) (up to 88.75% in nest 10), Cynophalla retusa (Capparaceae) (up to 84.74% in nest 7), Solanum type 2 (Solanaceae) (up to 84.83% in nest 9), Prosopis (Fabaceae, subfamily Mimosoideae) (up to 71.24% in nest 6), Ximenia americana (Ximeniaceae), type Senegalia praecox (Fabaceae, subfamily Mimosoideae), Anisocapparis speciosa (Capparaceae), Sideroxylon obtusifolium (Sapotaceae), Bulnesia sarmientoi (Zygophyllaceae), and Albizia inundata (Fabaceae, subfamily Mimosoideae) (Table II). This order varied when averaging these values for all nests together (N values of Table IV) (Prosopis, Solanum type 2, Cynophalla retusa, type Schinopsis, etc.).
When considering their relative contribution of biomass (i.e., the relative volume), Albizia inundata (Fabaceae, subfamily Mimosoideae) was detected as a highly represented resource in four out of the nine nests (Table II). Significant differences (p < 0.05) were found among direct pollen count and relative volume in six out of the nine pollen provision samples (Table II). These samples were dominated by grains of smaller absolute volume (such as Solanum type 2 in nest 9 or Ximenia americana in nest 8), medium grains of Prosopis followed by scarce amounts of large polyads of Albizia inundata or many small grains (Table II). In contrast, in the remaining three samples, the direct pollen count and relative volume were not significantly different (p ≥ 0.05) as they were dominated by grains of small volume of > 3000 μm3 such as Cynophalla retusa (nest 7) and type Schinopsis (nest 10) or by medium volume grains of the types Senegalia praecox and Prosopis (nest 11).
A total of 24 pollen types from 16 families were present in honey samples, from which 13 types (from 10 families) were abundantly represented. In decreasing order of their maximum value of direct pollen count, they were type Maytenus (Celastraceae) (up to 95.01% in nest 5), Cynophalla retusa (up to 64.41% in nest 7), Prosopis (up to 63.76% in nest 4), type Schinopsis (up to 53.89% in nest 10), Solanum type 2, Anisocapparis speciosa, type Senegalia praecox, Bulnesia sarmientoi (Zygophyllaceae), Ximenia americana, Ziziphus mistol (Rhamnaceae), Apocynoideae (Apocynaceae), Capparicordis/Anisocapparis (Capparaceae), and Pisonia zapallo (Nyctaginaceae) (Table II).
3.2 Absolute volume of pollen foraged
Absolute volume (mean ± SD) varied from 11,432 ± 5021 μm3 for Fridericia dichotoma monads (Bignoniaceae) or from 45,843 ± 9298 μm3 for the Albizia inundata polyads (Fabaceae, Mimosoideae) to 565 ± 63 μm3 for Ximenia americana (Ximeniaceae) (Table IV). The greatest volume values recorded were from Fabaceae (Mimosoideae), Bignoniaceae, Loranthaceae and Nyctaginaceae, while the smallest from Rhamnaceae, Celastraceae, Solanaceae, Zygophyllaceae, Fabaceae (Mimosoideae) and Ximeniaceae (Table IV). Only two of the five cytoplasmic volume categories proposed by Vossler (2015a) were identified in the diet: small and medium (Table IV). From the total of 23 pollen types or pollen species measured, 18 of them were small (565 ± 63 to 6433 ± 718 μm3) and five medium (8294 ± 1118 to 45,843 ± 9298 μm3). Taking into account the pollen types most abundant in the diet (ten types), the small pollen volumes were represented by seven pollen types while the medium ones by three (Table IV).
3.3 Diversity (H′) and evenness (J′) indices in the diet of Melipona orbignyi (Table II)
The highest value of H′ and J′ for pollen samples was from nest 4 (high richness and little variation in abundances). And, the highest ones for honey samples were from different nests (high richness in honey of nest 8 and high evenness in nests 1 and 11) (Table II). Taking into account all nests of M. orbignyi, mean H′ values were 0.97 ± 0.53 and 0.92 ± 0.42 for pollen provisions (for direct count and relative volume) and 1.17 ± 0.40 for honey, while J′ were 0.45 ± 0.13, 0.45 ± 0.15, and 0.55 ± 0.19, respectively.
3.4 The value of importance of species providers of nectar and pollen resources
According to this index, the most important nectar resources were type Maytenus (SI = 20.79), Prosopis (18.73), Cynophalla retusa (6.72) and Ziziphus mistol (3.32) (Table III), while the most important pollen resources were Prosopis (27.73), Cynophalla retusa (7.90), Solanum type 2 (6.57), and type Schinopsis (3.93) (Table III).
3.5 The index of relative importance of pollen hosts
The most important pollen types according to IRI values were Prosopis (IRI = 3354), due to its high constancy across the nests (F = 88.89%; i.e., it was found in eight out of the nine nests) and its high abundance in nests (N = 31.19%). It was followed by Albizia inundata (IRI = 1999) due mainly to its presence in approximately half of the samples (F = 55.56%) and its polyads which are the largest in absolute volume value (45,843 ± 9298 μm3; Table IV) of all the pollen types foraged, and thus, it has a high V value (34.46%) (Table IV). Similar to Prosopis, Cynophalla retusa (IRI = 921) and Solanum type 2 (IRI = 693) showed high F followed by N values, but low V values (Table IV). They were followed by type Senegalia praecox (574), type Schinopsis (509), Sideroxylon obtusifolium (322), Anisocapparis speciosa (317), Ximenia americana (292), Pisonia zapallo (203), etc. The lowest value was found for Trithrinax schizophylla (IRI = 18) (Table IV).
4 Discussion
4.1 Assessing the most important pollen resources in the bee diet
In the present study, a comparison using different types of calculations was provided showing that the importance of floral resources in the bee diet depends on the calculation used. Such comparison is usually not performed in entomopalynological studies. It is also an important issue to be taken into account in diet assessment of the different Anthophila. For instance, the broadly used direct pollen count showed that the order of the four most important pollen resources in the diet of Melipona orbignyi was type Schinopsis, Cynophalla retusa, Solanum type 2 and Prosopis; the relative volume showed that they were type Schinopsis, Prosopis, Cynophalla retusa and Solanum type 2; the value of importance of species (SI) showed that they were Prosopis, Cynophalla retusa, Solanum type 2 and type Schinopsis; and the IRI showed that they were Prosopis, Albizia inundata, Cynophalla retusa and Solanum type 2. Among the calculations performed for pollen resources, the IRI is the most complete and objective, as it takes into account the relative abundance of each pollen type, their relative biomass and constancy of usage across the nests in a single formula (Table IV). For example, if only N values were used, the importance of Albizia inundata would have been underestimated due to its low abundance among samples, and type Schinopsis and Ximenia americana overrated in the diet of M. orbignyi for their high abundance in samples but low volume and/or frequency. If only V values were used, the importance of abundant resources such as Solanum type 2 and Ximenia americana would have been underestimated for their low volume and those hardly foraged or contaminants such as Fridericia dichotoma and type Vachellia aroma overrated for their large volume. If only F values were used, the importance of type Schinopsis would have been underestimated as it was present in only three samples and that of Sideroxylon obtusifolium overrated as it was present in six nests but abundant only in one.
Some bee diet studies detected the importance of pollen resources for stingless bees by means of the relative volume considering that they are providers of high cytoplasm biomass, such as in the case of Pisonia zapallo (Nyctaginaceae) and Salta triflora (Polygonaceae) in the diet of Geotrigona argentina (Vossler et al. 2010), of Caesalpinia paraguariensis (Fabaceae, Caesalpinioideae), Sapium haematospermum (Euphorbiaceae) and Salta triflora in Tetragonisca fiebrigi (Vossler 2013; Vossler, submitted) and of Parkinsonia (Fabaceae, Caesalpinioideae) in M. orbignyi (the present study) in the Chaco region. If only direct pollen count had been calculated, they would not have been considered as an important resource for these stingless bees; this has also been reported for Apis mellifera by Biesmeijer et al. (1992). In conclusion, the relative volume should be more frequently used for assessing bee diets than only the direct pollen count as abundant pollen types could be assumed to be the most important in a bee diet, but when taking in consideration their relative volume, it could be otherwise (Buchmann and O’ Rourke 1991; O’Rourke and Buchmann 1991; Silveira 1991; Biesmeijer et al. 1992). Similarly, diversity and evenness indices calculated from volume estimates showed a different pattern of resource usage by Apis in Surinam than those from direct pollen counts (Biesmeijer et al. 1992).
Over and above the importance of volume, direct pollen count continues being the most important calculation as it provides the first quantitative information obtained directly from the samples and is therefore the only calculation performed in most bee diet studies. Moreover, it is the fastest way to estimate foraging intensity and floral preference. On the other hand, the estimation of the relative volume requires the measurement of pollen sizes preferably from unacetolyzed grains, which implies additional tasks to that of the direct pollen count. Although there is some inaccuracy in the estimation of pollen volume based on linear measurements because the shape of many pollen types does not conform to standard geometric figures, the calculation of relative volume based on such shapes confers a more accurate value than that obtained by direct pollen count alone. Although frequency is often used in combination with direct pollen count in honey studies (Lieux 1981; Cabrera 2006; Caccavari and Fagúndez 2010; Obregón and Nates-Parra 2014), it is not often enough used in pollen resource studies. In the present study, frequency and direct pollen count were combined in a formula (SI) according to Nates-Parra et al. (2013), without the need of using the relative volume, showing different results on the most important pollen resources (the SI underestimated Albizia inundata as this index does not consider pollen volume values).
4.2 Forest nectar resources foraged
The SI was useful to objectively determine the most important honey plants for M. orbignyi: type Maytenus, Prosopis, Cynophalla retusa and Ziziphus mistol, followed by Solanum type 2, type Senegalia praecox, Bulnesia sarmientoi and others. Different to IRI, this index does not consider the relative volume in its formula as pollen grain volume is irrelevant in the nectar diet. Therefore, SI is appropriate to assess the importance of nectar resources present in honey. The types Schinopsis, Anisocapparis speciosa and Ximenia americana from honey of nests 8 and 10 were probably contaminants during sampling and therefore discarded from this analysis. Solanum (ca. 2000 species) is recorded as having nectarless flowers with only pollen rewards in poricidal anthers (Vogel 1978; Symon 1979); however, it was found in pure honey samples of M. orbignyi (nest 1) and other Melipona in the Neotropics (Kleinert-Giovannini and Imperatriz-Fonseca 1987; Wilms and Wiechers 1997; Obregón and Nates-Parra 2014). Although the other meliponine genera are capable of robbing residual pollen grains found in Solanum petals and anther tips after being foraged by vibratile bees (Bezerra and Machado 2003), Solanum was not recorded in any type of provision of the non-buzzing bees Geotrigona and Tetragonisca from the Chaco dry forest (Vossler et al. 2010, 2014; Vossler 2013; Vossler, submitted). However, this pollen type has been recorded abundantly (> 10%) in a few honey samples of the non-buzzing bee Apis mellifera (Cabrera 2006; Caccavari and Fagúndez 2010). The abundance of this nectarless resource in honey samples of highly eusocial bees is possible because they can forage on non-Solanum nectar contaminated with Solanum pollen released during buzzing. And, as many researchers reported Solanum presence in pure honey samples, another alternative is the contamination within the colony before their sampling. However, it would be interesting to determine other causes of nectar and/or honey contamination, as they might occur at any time from the moment of foraging to honey sampling. It is relevant to mention the abundance of other pollen types from nectarless melittophilous species belonging to Mimosa, Acacia, Miconia, Spondias, some Arecaceae, Papaver, Sambucus, Cistus, etc., in stingless bee and Apis honeys, which can lead to misidentification of their botanical origin (Maurizio 1951; Roubik and Moreno Patiño 2013).
It is also worth mentioning that an association between highly nectariferous plants such as Prosopis, Maytenus and Ziziphus and high protein values of their pollen grains has been reported (Vossler 2015b) which could be related to the presence of Solanum in honeys.
4.3 Utilization of pollen and nectar resources by colonies of M. orbignyi
Melipona orbignyi colonies used a broad spectrum of pollen (25 pollen types of which 10 were intensively used) and nectar resources (24 of which 13 were intensively used). Concerning the usage of pollen and nectar resources, it was variable across colonies (high and low H′ and J′ values regardless the season) (Table II). Except for two spring nests (4 and 5) (Table II), H′ and J′ values of each nest were higher in honey than in pollen suggesting that M. orbignyi exploited differently nectar and pollen resources (nectar was mainly foraged from a higher number of plants and/or in a more homogeneous way (three to five sources predominated) than pollen (two sources) (Table II)). On the contrary, generally higher H′ values were recorded in pollen than honey in Geotrigona and Tetragonisca in this same forest (Vossler et al. 2010; Vossler 2013; Vossler, submitted).
Although many pollen types were present in each sample analyzed, only one, two or up to three were dominant. This foraging pattern (many plants foraged, but only a few abundantly represented) has already been observed in the diet of the studied meliponine genera and associated with the recruitment behavior highly developed in stingless bees (Ramalho et al. 1985, 1989, 2007; Kleinert-Giovannini and Imperatriz-Fonseca 1987; Ramalho 1990; Wilms and Wiechers 1997; Carvalho et al. 2001; Oliveira et al. 2009; Vossler et al. 2010; Hilgert-Moreira et al. 2013; Vossler 2018). Despite the fact that the total of 30 pollen types were foraged in both types of provisions in M. orbignyi, this genus is recorded as foraging on the lowest diversity of resources among stingless bees (Ramalho et al. 1985), which is believed to be caused by their small populated colonies of approximately 500–1000 individuals (i.e., there is a low exploration of floral resources) (Sommeijer et al. 1983; Ramalho et al. 1989), high efficiency in recruitment behavior (Ramalho et al. 1989, 2007; Hrncir et al. 2000; Jarau et al. 2000, 2003) and/or floral preferences (i.e., innate preferences instead of random exploitation of floral resources) (Ramalho et al. 2007).
4.4 Flower types associated with Melipona in the Chaco dry forest inferred from palynological data
There is sufficient evidence to support the hypothesis that floral traits associated to male structures (heteranthery, poricidal dehiscence, absence of floral nectaries and enantiostyly) are converged in different plant lineages as response to similar selective pressures modulated by pollinators and/or pollen thieves to avoid excessive removal of pollen from anthers (Hargreaves et al. 2009; Vallejo-Marín et al. 2010). These flower traits are present among the numerous forest species of the Chaco vegetation. However, among them, only the poricidal dehiscence seems to be associated with the buzzing behavior performed by many lineages of bees, among them the genus Melipona (Ramalho et al. 1989).
Buzzing behavior allowed Melipona orbignyi to legitimately collect large amounts of pollen from anthers of poricidal dehiscence from flowers of the ‘solanoid type’ sensu Vogel (1978) (Figure 1d), as it was the case of pollen grains of small volume of Solanum type 2 (Figure 1g). The entomopalynological studies carried out in the dry Chaco showed that these pollen resources were not foraged for the non-buzzing meliponines Geotrigona and Tetragonisca, not even in the illegitimate way. For instance, Bezerra and Machado (2003) demonstrated the effective pollination in Solanum by means of buzzing by Melipona scutellaris and that other stingless bee genera are only pollen robbers (Plebeia, Tetragonisca, Trigona s. str.). Many other studies also supported buzzing pollination by Melipona species in other poricidal anther flowers (Miconia, Tibouchina) (Renner 1989) as well as in flowers with longitudinally dehiscent anthers (Swartzia, Myrcia, Gomidesia) (Lopes and Machado 1996; Fidalgo and Kleinert 2009).
Other floral shapes associated to the predominant pollen grains found in the provisions of M. orbignyi were ‘brush type’ (sensu Vogel 1978) and the generalized small pale flowers (Figure 2). Brush flowers with numerous stamens massed like a ‘shaving brush’ have been reported by Buchmann (1985) as being associated with buzzing behavior in bees often together with the absence of floral nectar, either having poricidal (Bixa, Cochlospermum) or longitudinally dehiscent anthers (Rosa, Rubus, Argemone). The prevalence of Fabaceae Mimosoideae (Albizia inundata, Acacia s.l. and Prosopis) (Figure 2a, b) and Capparaceae in pollen provisions of M. orbignyi could be due to buzzing in their brush flowers with longitudinally dehiscent anthers. Although Prosopis and Capparaceae also were dominant in pollen samples of Geotrigona and Tetragonisca in the same forests, Albizia and Acacia s.l. were only found in Melipona samples.
An especial case requiring of further study is that of Ximenia americana whose showy hairs in petals might simulate stamen filaments possibly deceiving M. orbignyi from legitimate brush flowers. This resource was observed only in pollen provisions (and in one contaminated honey). The tight association between bees of the genus Melipona and brush flowers is here supported which would help to understand the high occurrence of Myrtaceae in provisions of tropical Melipona species documented in diverse entomopalynological studies (Ramalho et al. 1989, 2007; Marques-Souza et al. 1995; Marques-Souza 1996; Wilms and Wiechers 1997; Antonini et al. 2006; Luz et al. 2011; Hilgert-Moreira et al. 2013; Ferreira and Absy 2015). Both Myrtaceae (brush flowers) and Melastomataceae (those species with poricidal anthers) are pollen families usually associated with Melipona in tropical areas, but its absence in samples from the dry Chaco forest is due to they are not found in the area.
The generalized small pale flowers were here represented by the type Schinopsis (corresponding to Schinopsis and/or Schinus species) (Figure 2c) and Sideroxylon obtusifolium. They have tiny open nectar flowers of pale colors (greenish, yellowish and white) that bloom massively, a floral syndrome commonly found in tropical forests, and that has been strongly associated with generalist bees such as Meliponini, Halictidae and other small-sized generalist pollinators that concentrate their foraging on tree crowns (Bawa 1980, 1990; Wilms et al. 1997; Ramalho 2004; Monteiro and Ramalho 2010). This kind of generalized flower is among the most foraged by non-buzzing meliponine genera (Scaptotrigona, Tetragonisca and Geotrigona) in the Chaco dry forest (Vossler 2015a). Other pollination syndromes with specific flower morphology of minor importance associated with buzzing bees were gullet-shaped zygomorphic flowers of Angelonia pubescens (Plantaginaceae s. str. (Vogel and Machado 1991) and Pedicullaris (Orobanchaceae s. str.) (Buchmann 1985)), but they were not detected in the present study.
4.5 Recommendations on forest management for native pollinator conservation
Native pollinators as pollen vectors have become of interest as pollination of many forest species is in risk due to isolation and degradation of forest patches by human activities, as the increasingly observed in the Chaco region of South America (Aizen and Feinsinger 1994a, 1994b; Ferreyra et al. 2007; Gasparri and Grau 2009; Bessega et al. 2012; Piquer-Rodríguez et al. 2015). Due to their important role in forest dynamic and that many problems have been detected concerning the actual Chaco forest management, their need of conservation has recently been highlighted and many good practices incorporated for a sustainable management of forests integrating cattle ranching (Aprile et al. 2016). It is important to point out that the recommendations here proposed apply to class 2 or yellow zone forests (forests for sustainable usage) according to the Argentine Forest Law of 2007 (Ley de Presupuestos Mínimos de Protección Ambiental de los Bosques Nativos 26.331).
Palynological studies and the usage of multiple calculations to assess bee diets, such as here performed, are good tools to objectively detect the most important floral resources for bees in natural ecosystems, which is needed to establish good forest management practices for pollinator conservation and to restore very anthropized forests. Moreover, although sampling was not systematically performed in the present study, the samples here analyzed are important as they were taken from continuous native dry forest in the core of the Chaco region (1,000,000 km2) which is increasingly being fragmented. Therefore, these samples will have an added value as they show how the flora-native bee interaction was prior to the fragmentation, useful information in forest conservation in anthropized areas.
Melipona species are especially important in forest conservation as they are capable of cross-pollination among relatively distant forest patches (Roubik and Aluja 1983; Araújo et al. 2004) and the pollination of resources not used by other stingless bees such as solanoid flowers (Bezerra and Machado 2003). The IRI showed that medium- and small-sized trees and shrubs (Prosopis, Albizia, Cynophalla) as well as herbaceous or shrubby Solanum were the most important resources for M. orbignyi followed by Senegalia, Schinopsis, Sideroxylon, Anisocapparis, Ximenia, etc., being their preservation a good practice to promote foraging and conservation of this bee species. Good forest management practices for pollinator conservation native to the Chaco forest proposed by Aprile et al. (2016) included the keeping of undisturbed forest patches composed of plant species used for nesting and foraging while the surrounding forest resources are managed in a sustainable way. The species of these patches included trees, shrubs and herbs composing the three strata, those with soft trunks such as Cactaceae, Ceiba and Phytolacca and the dead trunks and those blooming in different seasons thus preserving floral availability during long periods. These practices aim at preserving nesting substrates and sites of diverse groups of pollinators, which usually are extracted and discarded as useless for logging. Other good practices included the maintenance of water bodies, and patches with undisturbed soil and open vegetation. Aprile et al. (2016) noticed that one of the main threats to native pollinators in the Chaco region is the low flower availability in homogeneous forests and/or during certain months, which is intensified in sites with selective extraction of lower strata (a few tree species are selectively being maintained for future logging and the remaining, mainly shrubs, deforested during understory ‘cleaning’). Brosi (2009) reported that in tropical forests (continuous forests and patches of diverse size), stingless bee composition was most strongly related to plant species richness, only weakly to forest cover and not related to blooming plant abundance. Other threats noticed by Aprile et al. (2016) were the low water availability during certain months, agrochemical contamination from nearby crops and different degree of competition with the exotic ‘honey bee’ Apis mellifera for floral resources. The access of A. mellifera to floral resources seems to be facilitated in forest fragmentation conditions in detriment of the access by native flower visitors (Aizen and Feinsinger 1994b).
It is important to mention that many Chaco forest species are used by stingless bees for both nesting and foraging (Vossler 2012). It has been reported nesting by M. orbignyi in large and medium living tree trunks of three Prosopis species, Bulnesia sarmientoi and Ziziphus mistol (Vossler 2012), and these species also are abundant in its pollen and honey provisions. Another important food source is Solanum, with poricidal anthers in solanoid type flowers and high protein levels (Roulston et al. 2000), preferred by Melipona bees along the Neotropics (Ramalho et al. 1989). Both Prosopis and Solanum are rich genera, and they are abundant in the Chaco forest providing a long flowering period for foraging (Vossler 2013). They are mainly composed of pioneer species growing in open and disturbed areas such as deforested sites, abandoned crops, forest patch edges and riparian habitats, and they are very commonly found in grazing sites (obs. pers.). Although these floral resources are preferred by M. orbignyi and abundant in continuous and fragmented forests, Prosopis trees (P. alba, P. nigra, P. kuntzei, P. ruscifolia, P. vinalillo) are being intensively exploited as they are very reputable for their hardwood mainly for furniture. Moreover, the expansion of soybean production and intensified cattle ranching are the main causes of Chaco dry forest loss (Gasparri and Grau 2009; Piquer-Rodríguez et al. 2015). This region experienced a dramatic acceleration of fragmentation and loss of connectivity: during 1977–2002, about 20,000 km2 were deforested, while almost 40,000 km2 were deforested in 2002–2010 (Piquer-Rodríguez et al. 2015). This equals a rate of roughly 4750 km2/year (> 0.5 km2/h) over the last decade, being more than double the rates from the 1990s and more than three times the rates from the 1980s (Piquer-Rodríguez et al. 2015).
Furthermore, Ferreyra et al. (2007) showed that most of genetic variability of Prosopis alba and other related Prosopis species occurs within populations and regional differentiation is limited. Bessega et al. (2012) highlighted the need of conserving short distant forest patches to avoid the effects of inbreeding and drift within populations of P. alba. Due to its limited pollen and seed dispersal, Bessega et al. (2012) proposed a strategy to preserve in situ diversity of P. alba compatible with a rational usage of resources which would imply the conservation of patches connected by pollinators and seed dispersers in agroforestry systems combined with livestock breeding. Due to the numerous elements of the medium and low forest strata in pollen and nectar provisions found in the present study (the most important being Prosopis, Albizia, Cynophalla, Solanum, Maytenus, Ziziphus, Senegalia, Anisocapparis, Ximenia), the keeping of undisturbed patches in the cattle ranching–managed forests is therefore a good management practice to preserve pollen vectors such as M. orbignyi. Furthermore, the dominant high stratum elements of the Chaco forest were also found to be important in its diet, and they have been historically intensively exploited in a selective way mainly for extraction of tannin and railway sleepers in the case of ‘quebrachos colorados’ (Schinopsis balansae, S. lorentzii and S. heterophylla) (Zarrilli 2008; Barberis et al. 2012) and the pleasantly smelling essential oil by perfume and soap industry in the case of ‘palo santo’ (Bulnesia sarmientoi) (Waller et al. 2012), and therefore, they are nowadays of reduced height and/or are scarce in many areas resulting in lower forests (Zarrilli 2008; obs. pers.). The Schinopsis species and Bulnesia sarmientoi grow together with another hardwood dominant tree also of slow growth: the ‘quebracho blanco’ (Aspidosperma quebracho-blanco), which has been selectively exploited for coal production (Ezcurra 1981). Populations of these dominant trees have been isolated as forest fragmentation reached unprecedented levels (Gasparri and Grau 2009; Piquer-Rodríguez et al. 2015). Similarly to what was reported for Prosopis species, gene flow is necessary to avoid the negative effects of population isolation in Schinopsis balansae, as Vesprini et al. (2011) showed the importance of the presence of staminate individuals near pistillate ones to provide genetic variability in reduced populations. On the other hand, A. quebracho-blanco is apparently pollinated by nocturnal moths by means of deceit and whose floral rewards are provided by other sympatric plant species (Lin and Bernardello 1999), which indicates the need to preserve many Chaco forest elements to ensure the reproductive success of this dominant tree. Information on breeding system and effect of fragmentation on B. sarmientoi and the other Schinopsis species are yet unknown, as well as on most Chaco forest species important in the diet of M. orbignyi.
These dominant large trees characteristic of the Chaco region forest (and which provide local names to these forests: ‘quebrachal’ and ‘palosantal’) have been exploited for more than 140 years (Zarrilli 2008). For instance, White (1957) reported that the annual production of tannin extracts from Schinopsis species reached 200,000–250,000 t from natural stands of timber from Argentina and Brazil and that their cultivation (reforestation) was not organized (or simply not carried out (Valentini 1960)). During these years, although saplings were observed growing among grasses after logging, natural reforestation was impeded by browsing and trampling by cattle and burning of the fields (Valentini 1960), practices still carried out. However, current threats include complete deforestation using bulldozers, burning and finally the extensive monoculture of soybean crops. In red zones or class 1 forests (fully protected forests of non-commercial usage, according to the Argentine Forest Law), the clandestine deforestation is currently a common practice, being the Chaco forest severely endangered. This devastation is also valid for the palosantal forest of B. sarmientoi, which timber primary production in Argentina and Paraguay soared from less than 500 t of logs in 2000 to 35,000 t annually in the last years, and Argentina seems to be the main producer, with more than 20,000 t of timber harvested each year (Waller et al. 2012). Argentina is currently the major exporter of B. sarmientoi timber, while Paraguay is the main producer of essential oil for the international perfume industry (Waller et al. 2012).
Although large forest fragments provide better habitats for many forest species, the small ones are also important to be preserved particularly in cases when they function as stepping stones for connecting large forest remnants (Piquer-Rodríguez et al. 2015). For instance, Torrella et al. (2015) showed that small fragments in the Three Quebrachos Forest of the Central Chaco presented the same density and size-class structure than the larger ones in the dominant trees Schinopsis balansae, Aspidosperma quebracho-blanco, Prosopis kuntzei among others, but these forest remnants are strongly threatened by agriculture expansion. In this context, the usage of non-timber forest resources, such as the meliponiculture, would allow the gene flow by pollen dispersal among isolated individuals and patches and therefore enhance the resistance to the negative effects of the fragmentation. On the other hand, it is worth mentioning that fragments isolated by distances longer than the longest flight range (2 to 2.4 km in Melipona) likely produce the isolating of Melipona colonies which are particularly sensitive to the effects of genetic drift due to homozygosity in the Xo sex determination locus (Araújo 2000). Some Melipona species from Brazil appeared to be affected by deforestation as they were present mainly in areas where the forest was more undisturbed (Brown and Albrecht 2001). Stingless bees of medium (Scaptotrigona, Geotrigona, Lestrimelitta) and small sizes (Tetragonisca, Plebeia) are more susceptible to negative effects of population isolation as their maximum flight range is more reduced (Araújo et al. 2004). Moreover, extinction of local populations of Meliponini due to loss of alleles has been reported (Carvalho et al. 1995). For the above exposed, fragment connectivity is needed for conservation of plant and stingless bee population structure.
Among pollinators, the management of stingless bees is a good option as they are successfully reared in boxes and containers of different designs in tropical and subtropical areas of the world (Venturieri et al. 2003), and most species are suitable for meliponiculture in the dry Chaco region (Vossler 2012). Although this activity still remains incipient in the Chaco forest, these bees could be reared in forest understory in the same way as Apis mellifera, but adapted for unfavorable climate conditions such as frost and insolation. Hive densities should be low in small patches, to avoid floral resource competition and massive robbing as the occurring between Apis and Scaptotrigona in this dry forest (Vossler et al., in prep.). A good management of forest would include the rearing of several native stingless bee species both in their natural nesting holes (mainly large living tree trunks) to preserve mother colonies and in boxes for a hygienic and careful extraction of their products (honey, pollen, cerumen), reproduction of colonies and other practices.
Nowadays, intensive rearing of Tetragonisca fiebrigi and Scaptotrigona jujuyensis for honey production in a large area of the Chaco forest has recently been proposed, but a balance between conservation and economic aims is needed to avoid an excessive nest extraction directly from native forest trees (as it happened in Misiones province, a neighboring region of Northeastern Argentina, where Tetragonisca honeys reached very high prices). The intensive management of M. orbignyi has not yet been proposed, but the present study provides useful information on the floral resources used by colonies sampled in the continuous native forest, which is important for good colony management and for restoring degraded forest patches. For instance, the most important resource for the Chaquenian Melipona species is Prosopis, which are colonizing species known to facilitate the recolonization and establishment of other plants (Cesca et al. 2012).
In the present study, Melipona orbignyi foraged on a large number of forest species and likely helped to pollinate them, which is needed for their conservation and the maintenance of healthy forests. Although pollination effectiveness by M. orbignyi was not assessed, it is important to be considered for further studies, mainly on threatened species of this forest. Although a vast number of crops are suitable for pollination using this species and particularly Solanum (tomato, eggplant) due to its buzzing behavior, this has not yet been implemented in the region (Vossler et al. 2018).
References
Adámoli J., Sennhauser E., Acero J.M., Rescia A. (1990) Stress and disturbance: vegetation dynamics in the dry Chaco region of Argentina. J. Biogeogr. 17, 491–500.
Aizen M.A., Feinsinger P. (1994a) Forest fragmentation, pollination, and plant reproduction in a Chaco dry forest, Argentina. Ecology 75, 330–351.
Aizen M.A., Feinsinger P. (1994b) Habitat fragmentation, native insect pollinators, and feral honey bees in Argentine 'Chaco Serrano'. Ecol. Appl. 4, 378–392.
Antonini Y., Costa R.G., Martins R.P. (2006) Floral preferences of a neotropical stingless bee, Melipona quadrifasciata Lepeletier (Apidae: Meliponina) in an urban forest fragment. Braz. J. Biol. 66, 463–471.
Aprile G., Periago M.E., Miñarro F.O. (2016) La fauna y los silvopastoriles del Chaco. Boletín técnico. Fundación Vida Silvestre Argentina, Buenos Aires, Argentina. https://www.vidasilvestre.org.ar/sala_redaccion/opublicaciones/?15661/Boletn-tcnico---La-fauna-y-los-silvopastoriles-del-Chaco.
Araújo E.D. (2000) Extinção em populações do gênero Melipona (Hymenoptera: Meliponinae): efeito do tamanho populacional e da produção de machos por operárias. Naturalia 25, 287–299.
Araújo E.D., Costa M., Chaud-Netto J., Fowler H.G. (2004) Body size and flight distance in stingless bees (Hymenoptera: Meliponini): inference of flight range and possible ecological implications. Braz. J. Biol. 64, 563–568.
Barberis I.M., Mogni V.Y., Oakley L.J., Alzugaray C., Vesprini J.L., Prado, D.E. (2012) Schinopsis balansae Engl. (Anacardiaceae). Kurtziana 37, 59–86.
Bawa K.S. (1980) Evolution of dioecy in flowering plants. Annu. Rev. Ecol. Syst. 11, 15–39.
Bawa K.S. (1990) Plant-pollinator interactions in tropical rainforest. Annu. Rev. Ecol. Syst. 21, 399–422.
Bessega C., Pometti C.L., Ewens M., Saidman B.O., Vilardi J.C. (2012) Strategies for conservation for disturbed Prosopis alba (Leguminosae, Mimosoidae) forests based on mating system and pollen dispersal parameters. Tree Genet. Genomes 8, 277–288.
Bezerra E.L.S., Machado I.C. (2003) Biologia floral e sistema de polinização de Solanum stramonifolium Jacq. (Solanaceae) em remanescente de Mata Atlântica, Pernambuco. Acta Bot. Bras. 17, 247–257.
Biesmeijer J.C., Van Marwijk B., Deursen K., Punt W., Sommeijer M.J. (1992) Pollen sources for Apis mellifera L. (Hym., Apidae) in Surinam, based on pollen grain volume estimates. Apidologie 23, 245–256.
Brosi B.J. (2009) The complex responses of social stingless bees (Apidae: Meliponini) to tropical deforestation. For. Ecol. Manag. 258, 1830–1837.
Brosi B.J., Daily G.C., Shih T.M., Oviedo F., Durán G. (2008) The effects of forest fragmentation on bee communities in tropical countryside. J. Appl. Ecol. 45, 773–783.
Brown J.C., Albrecht C. (2001) The effect of tropical deforestation on stingless bees of the genus Melipona (Insecta: Hymenoptera: Apidae: Meliponini) in central Rondonia, Brazil. J. Biogeogr. 28, 623–634.
Buchmann S.L. (1985) Bees use vibration to aid pollen collection from non-poricidal anthers. J. Kansas Entomol. Soc. 58, 517–525.
Buchmann S.L., O’ Rourke M.K. (1991) Importance of pollen grain volumes for calculating bee diets. Grana 30, 591–595.
Cabrera M.M. (2006) Caracterización polínica de las mieles de la provincia de Formosa, Argentina Rev. Museo Argent. Cienc. Nat. 8, 135–142.
Caccavari M.A., Fagúndez G.A. (2010) Pollen spectrum of honeys from the Middle Delta of the Paraná River (Argentina) and their environmental relationship. Span. J. Agric. Res. 8, 42–52.
Camargo J.M.F., Pedro S.R.M. (2013) Meliponini Lepeletier, 1836. In: Moure J.S., Urban D., Melo G.A.R. (Orgs.), Catalogue of Bees (Hymenoptera, Apoidea) in the Neotropical Region - online version. Available at http://www.moure.cria.org.br/catalogue. Accessed Nov/24/2017
Cane J.H., Sipes S. (2006) Characterizing floral specialization by bees: analytical methods and a revised lexicon for oligolecty. In: Waser N.M., Ollerton J. (Eds.), Plant-Pollinator Interactions. From specialization to generalization. The University of Chicago Press, Chicago and London, pp. 99–122.
Carvalho G.A., Kerr W.E., Nascimento V.A. (1995) Sex determination in bees. XXXIII. Decrease of xo hetero-alleles in a finite population of Melipona scutellaris (Apidae, Meliponini). Braz. J. Genet. 18, 13–16.
Carvalho C.A.L., Moreti A.C., Marchini L.C., Alves R.M., Oliveira P.C.F. (2001) Pollen spectrum of honey of “uruçu” bee (Melipona scutellaris Latreille, 1811). Rev. Bras. Biol. 61, 63–67.
Cesca E.M., Villagra P.E., Passera C., Alvarez J.A. (2012) Effect of Prosopis flexuosa on understory species and its importance to pastoral management in woodlands of the Central Monte Desert. Rev. Fac. Cienc. Agrarias UNCUYO 44, 207–219.
Cortopassi-Laurino M., Ramalho M. (1988) Pollen harvest by Africanized Apis mellifera and Trigona spinipes in São Paulo, botanical and ecological views. Apidologie 19, 1–24.
Cortopassi-Laurino M., Imperatriz-Fonseca V.L., Roubik D.W., Dollin A., Heard T., Aguilar I., Venturieri G.C., Eardley C., Nogueira-Neto P. (2006) Global meliponiculture: challenges and opportunities. Apidologie 37, 275–292.
Cortopassi-Laurino M., Zillikens A., Steiner J. (2009) Pollen sources of the orchid bee Euglossa annectans Dressler 1982 (Hymenoptera: Apidae, Euglossini) analyzed from larval provisions. Genet. Mol. Res. 8, 546–556.
Dalmazzo M., Vossler F.G. (2015) Assessment of the pollen diet in a wood-dwelling augochlorine bee (Halictidae) using different approaches. Apidologie 46, 478–488.
De Klerk P., Joosten H. (2007) The difference between pollen types and plant taxa: a plea for clarity and scientific freedom. Q. Sci. J. 56, 162–171.
Erdtman G. (1960) The acetolysis method, a revised description. Sven. Bot. Tidskr. 54, 561–564.
Ezcurra C. (1981) Revisión de las Apocináceas de la Argentina. Darwiniana 23, 367–474.
Ferreira M.G., Absy M.L. (2015) Pollen niche and trophic interactions between colonies of Melipona (Michmelia) seminigra merrillae and Melipona (Melikerria) interrupta (Apidae: Meliponini) reared in floodplains in the Central Amazon. Arthropod Plant Interact. 9, 263–279.
Ferreira P.A., Boscolo D., Carvalheiro L.G., Biesmeijer J.C., Rocha P.L.B., Viana B.F. (2015) Responses of bees to habitat loss in fragmented landscapes of Brazilian Atlantic Rainforest. Landsc. Ecol. 30, 2067–2078.
Ferreyra L.I., Bessega C., Vilardi J.C., Saidman B.O. (2007) Consistency of population genetics parameters estimated from isozyme and RAPDs dataset in species of genus Prosopis (Leguminosae, Mimosoideae). Genetica 131, 217–230.
Fidalgo A.O., Kleinert A.M.P. (2009) Reproductive biology of six Brazilian Myrtaceae: is there a syndrome associated with buzz-pollination? N. Z. J. Bot. 47, 355–365.
Gasparri N.I., Grau H.R. (2009) Deforestation and fragmentation of Chaco dry forest in NW Argentina (1972–2007). For. Ecol. Manag. 258, 913–921.
Hammer Ø., Harper D.A.T., Ryan P.D. (2008) PAST-Palaeontological Statistics, version 1.81.
Hargreaves A.L., Harder L.D., Johnson S.D. (2009) Consumptive emasculation: the ecological and evolutionary consequences of pollen theft. Biol. Rev. 84, 259–276.
Hart R.K., Calver M.C., Dickman C.R. (2002) The index of relative importance: an alternative approach to reducing bias in descriptive studies of animal diets. Wildl. Res. 29, 415–421.
Hilgert-Moreira S., Nascher C., Callegari-Jacques S., Blochtein B. (2013) Pollen resources and trophic niche breadth of Apis mellifera and Melipona obscurior (Hymenoptera, Apidae) in a subtropical climate in the Atlantic rain forest of southern Brazil. Apidologie 45, 129–141.
Hrncir M., Jarau S., Zucchi R., Barth F.G. (2000) Recruitment behavior in stingless bees, Melipona scutellaris and Melipona quadrifasciata. II. Possible mechanisms of communication. Apidologie 31, 93–113.
Hutcheson K. (1970) A test for comparing diversities based on the Shannon formula. J. Theor. Biol. 29, 151–154.
Jarau S., Hrncir M., Zucchi R., Barth F.G. (2000) Recruitment behavior in stingless bees, Melipona scutellaris and Melipona quadrifasciata. I. Foraging at food sources differing in direction and distance. Apidologie 31, 81–91.
Jarau S., Hrncir M., Schmidt V.M., Zucchi R., Barth F.G. (2003) Effectiveness of recruitment behavior in stingless bees (Apidae, Meliponini). Insect. Soc. 50, 365–374.
Joosten H., De Klerk P. (2002) What's in a name? Some thoughts on pollen classification, identification, and nomenclature in Quaternary palynology. Rev. Palaeobot. Palynol. 122, 29–45.
Kajobe R. (2006) Pollen foraging by Apis mellifera and stingless bees Meliponula bocandei and Meliponula nebulata in Bwindi Impenetrable National Park, Uganda. Afr. J. Ecol. 45, 265–274.
Kleinert-Giovannini A., Imperatriz-Fonseca V.L. (1987) Aspects of the trophic niche of Melipona marginata marginata Lepeletier (Apidae, Meliponinae). Apidologie 18, 69–100.
Lieux M.H. (1981) An analysis of Mississipi (U.S.A.) honey: pollen, color and moisture. Apidologie 12, 137–158.
Lin S., Bernardello G. (1999) Flower structure and reproductive biology in Aspidosperma quebracho-blanco (Apocynaceae), a tree pollinated by deceit. Int. J. Plant Sci. 160, 869–878.
Lopes A.V., Machado I.C.S. (1996) Biologia floral de Swartzia pickelii Killip ex Ducke (Leguminosae-Papilionoideae) e sua polinização por Eulaema spp. (Apidae-Euglossini). Rev. Bras. Bot. 19, 17–24.
Luz C.F.P., Fernandes-Salomão T.M., Lage L.G.A., Resende H.C., Tavares M.G., Campos L.A.O. (2011) Pollen sources for Melipona capixaba Moure & Camargo: an endangered Brazilian stingless bee. Psyche, 107303. https://doi.org/10.1155/2011/107303.
Marques-Souza A.C. (1996) Fontes de pólen exploradas por Melipona compressipes manaosensis (Apidae: Meliponinae), abelha da Amazônia Central. Acta Amazon. 26, 77–86.
Marques-Souza A.C., Absy M.L., Kerr W.E., Aguilera Peralta F.J. (1995) Pólen coletado por duas espécies de Meliponíneos (Hymenoptera: Apidae) da Amazônia. Rev. Bras. Biol. 55, 855–864.
Maurizio A. (1951) Pollen analysis of honey. Bee World 31, 1–5.
Monteiro D., Ramalho M. (2010) Abelhas generalistas (Meliponina) e o sucesso reprodutivo de Stryphnodendron pulcherrimum (Fabales: Mimosaceae) com florada em massa na Mata Atlântica, BA. Neotrop. Entomol. 39, 519–526.
Nates-Parra G., Roubik D. (1990) Sympatry among subspecies of Melipona favosa in Colombia and taxonomic revision. J. Kansas Entomol. Soc. 63, 200–203.
Nates-Parra G., Montoya P.M., Chamorro F.J., Ramírez N., Giraldo C., Obregón D. (2013) Origen geográfico y botánico de mieles de Apis mellifera (Apidae) en cuatro departamentos de Colombia. Acta Biol. Colombiana 18, 427–438.
Nunes-Silva P., Hrncir M., Imperatriz-Fonseca V.L. (2010) A polinização por vibração. Oecol. Australis 14, 140–151.
O’Rourke M.K., Buchmann S.L. (1991) Standardized analytical techniques for bee-collected pollen. Environ. Entomol. 20, 507–513.
Obregón D., Nates-Parra G. (2014) Floral preference of Melipona eburnea Friese (Hymenoptera: Apidae) in a Colombian Andean Region. Neotrop. Entomol. 43, 53–60.
Oliveira F.P.M., Absy M.L., Miranda I.S. (2009) Recurso polínico coletado por abelhas sem ferrão (Apidae, Meliponinae) em um fragmento de floresta na região de Manaus - Amazonas. Acta Amazon. 39, 505–518.
Papadakis J. (1973) La región chaqueña. Ecología, suelos, posibilidades agrícolas. Cienc. Investig. 29, 182–201.
Pielou E.C. (1977) Mathematical Ecology. Wiley, New York.
Pinkas L., Oliphant M.S., Iverson I.L.K. (1971) Food habits of albacore, bluefin tuna, and bonito in California waters. University of California, Department of Fish and Game. Fish. Bull. 152, 1–105.
Piquer-Rodríguez M., Torrella S., Gavier-Pizarro G., Volante J., Somma D., Ginzburg R., Kuemmerle T. (2015) Effects of past and future land conversions on forest connectivity in the Argentine Chaco. Landsc. Ecol. 30, 817–833.
Prado D.E. (1993) What is the Gran Chaco vegetation in South America? I. a review. Contribution to the study of flora and vegetation of the Chaco. V. Candollea 48, 145–172.
Ramalho M. (1990) Foraging by stingless bees of the genus Scaptotrigona (Apidae, Meliponinae). J. Apic. Res. 29, 61–67.
Ramalho M. (2004) Stingless bees and mass flowering trees in the canopy of Atlantic Forest: a tight relationship. Acta Bot. Bras. 18, 37–47.
Ramalho M., Imperatriz-Fonseca V.L., Kleinert-Giovannini A., Cortopassi-Laurino M. (1985) Exploitation of floral resources by Plebeia remota Holmberg (Apidae, Meliponinae). Apidologie 16, 307–330.
Ramalho M., Kleinert-Giovannini A., Imperatriz-Fonseca V.L. (1989) Utilization of floral resources by species of Melipona (Apidae, Meliponinae): Floral preferences. Apidologie 20, 185–195.
Ramalho M., Silva M.D., Carvalho C.A.L. (2007) Dinâmica de uso de fontes de pólen por Melipona scutellaris Latreille (Hymenoptera: Apidae): uma análise comparativa com Apis mellifera L. (Hymenoptera: Apidae), no Domínio Tropical Atlântico. Neotrop. Entomol. 36, 38–45.
Rech A.R., Absy M.L. (2011) Pollen sources used by species of Meliponini (Hymenoptera: Apidae) along the Rio Negro channel in Amazonas, Brazil. Grana 50, 150–161.
Renner S.S. (1989) A survey of reproductive biology in Neotropical Melastomataceae and Memecylaceae. Ann. Mo. Bot. Gard. 76, 496–518.
Roubik D.W. (2006) Stingless bee nesting biology. Apidologie 37, 124–143.
Roubik D.W., Aluja M. (1983) Flight ranges of Melipona and Trigona in tropical forest. J. Kansas Entomol. Soc. 56, 217–222.
Roubik D.W., Moreno Patiño J.E. (2013) How to be a bee-botanist using pollen spectra. In Vit P., Pedro S.R.M., Roubik D.W. (Eds.). Pot honey: A legacy of stingless bees (pp. 295–314). New York: Springer.
Roulston T.H., Cane J.H., Buchmann S.L. (2000) What governs protein content of pollen: pollinator preferences, pollen-pistil interactions, or phylogeny? Ecol. Monogr. 70, 617–643.
Schwarz H.F. (1932) The genus Melipona. Bull. Am. Mus. Nat. Hist. 63, 231–460, pls. I-X.
Silveira da F.A. (1991) Influence of pollen grain volume on the estimation of the relative importance of its source to bees. Apidologie 22, 495–502.
Sommeijer M.J., Rooy de G.A., Punt W., de Bruijn L.L.M. (1983) A comparative study of foraging behavior and pollen resources of various stingless bees (Hym., Meliponinae) and honeybees (Hym., Apinae) in Trinidad, West-Indies. Apidologie 14, 205–224.
Symon D. (1979) Sex forms in Solanum and the role of pollen collecting insects. In: Hawkes J.G., Lester R.N., Skelding A.D. (Eds.), The Biology and Taxonomy of the Solanaceae, Linnean Society Symposium Series No. 7, Academic Press, pp. 385–396.
Torrella S., Ginzburg R., Galetto L. (2015) Forest fragmentation in the Argentine Chaco: recruitment and population patterns of dominant tree species. Plant Ecol. 216, 1499–1510.
Ueira-Vieira C., Nunes-Silva C.G., Absy M.L., Costa Pinto M.F.F., Kerr W.E., Bonetti A.M., Carvalho-Zilse G.A. (2013) Pollen diversity and pollen ingestion in an Amazonian stingless bee, Melipona seminigra (Hymenoptera, Apidae). J. Apic. Res. 52, 173–178.
Valentini J.A. (1960) La reforestación con quebracho colorado y algunas normas silvícolas relacionadas con su aprovechamiento racional. Bonplandia 1, 51–69.
Vallejo-Marín M., Da Silva E.M., Sargent R.D., Barrett S.C.H. (2010) Trait correlates and functional significance of heteranthery in flowering plants. New Phytol. 188, 418–425.
Venturieri G.C., Raiol V.F.O., Pereira C.A.B. (2003) Avaliação da introdução da criação recional de Melipona fasciculata (Apidae: Meliponina), entre os agricultores familiares de Bragança - PA, Brasil. Biota Neotrop. 3, 1–7.
Vesprini J.L., Torres P., Ortiz J.P. (2011) Riqueza alélica y diversidad génica en dos poblaciones de Schinopsis balansae (Engl.). II Congreso Nacional de Protección y Manejo Sustentable de Bosque Nativo, Villaguay.
Villanueva-Gutiérrez R., Roubik D.W. (2004) Why are African honey bees and not European bees invasive? Pollen diet diversity in community experiments. Apidologie 35, 481–491.
Vogel S. (1978) Evolutionary shifts from reward to deception in pollen flowers. In: Richards, A.J. (Ed.), The pollination of flowers by insects, Academic Press, London, pp. 89–96.
Vogel S., Machado I.C. (1991) Pollination of four sympatric species of Angelonia (Scrophulariaceae) by oil-collecting bees in NE Brazil. Plant Syst. Evol. 178, 153–178.
Vossler F.G. (2012) Flower visits, nesting and nest defence behaviour of stingless bees (Apidae: Meliponini): suitability of the bee species for meliponiculture in the Argentinean Chaco region. Apidologie 43, 139–161.
Vossler F.G. (2013) Estudio palinológico de las reservas alimentarias (miel y masas de polen) de “abejas nativas sin aguijón” (Hymenoptera, Apidae, Meliponini): un aporte al conocimiento de la interacción abeja-planta en el Chaco Seco de Argentina. Ph.D Dissertation, Universidad Nacional de La Plata, La Plata, Argentina, http://sedici.unlp.edu.ar/handle/10915/32478. Accessed 23 May 2013.
Vossler F.G. (2015a) Small pollen grain volumes and sizes dominate the diet composition of three South American subtropical stingless bees. Grana 54, 68–81.
Vossler F.G. (2015b) Broad protein spectrum in stored pollen of three stingless bees from the Chaco dry forest in South America (Hymenoptera, Apidae, Meliponini) and its ecological implications. Psyche, 659538. https://doi.org/10.1155/2015/659538
Vossler F.G. (2018) Are stingless bees a broadly polylectic group? An empirical study of the adjustments required for an improved assessment of pollen diet in bees. In: Vit P., Pedro S.R.M., Roubik D.W. (Eds.), Pot-Pollen in Stingless Bee Melittology (pp. 17–28). Cham: Springer.
Vossler F.G., Tellería M.C., Cunningham M. (2010) Floral resources foraged by Geotrigona argentina (Apidae, Meliponini) in the Argentine Dry Chaco forest. Grana 49, 142–153.
Vossler F.G., Fagúndez G.A., Blettler D.C. (2014) Variability of food stores of Tetragonisca fiebrigi (Schwarz) (Hymenoptera: Apidae: Meliponini) from the Argentine Chaco based on pollen analysis. Sociobiology 61, 449–460.
Vossler F.G., Blettler D.C., Fagúndez G.A., Dalmazzo M. (2018) Stingless bees as potential pollinators in agroecosystems in Argentina: inferences from pot-pollen studies in natural environments. In: Vit P., Pedro S.R.M., Roubik D.W. (Eds.), Pot-Pollen in Stingless Bee Melittology (pp. 155–175). Cham: Springer.
Waller T., Barros M., Draque J., Micucci P. (2012) Conservation of the Palo Santo tree, Bulnesia sarmientoi Lorentz ex Griseb, in the South America Chaco Region. Med. Plant Conserv. 15, 4–9.
White T. (1957) Tannins: their occurrence and significance. J. Sci. Food Agric. 8, 377–385.
Wilms W., Ramalho M., Wendel L. (1997) Stingless bees and Africanized honey bees in the Mata Atlântica rainforest of Brazil. XXXth International Apicultural Congress of Apimondia, Antuérpia, pp. 167–170.
Wilms W., Wiechers B. (1997) Floral resource partitioning between native Melipona bees and the introduced Africanized honey bee in the Brazilian Atlantic rain forest. Apidologie 28, 339–355.
Zak M.R., Cabido M., Hodgson J.G. (2004) Do subtropical seasonal forests in the Gran Chaco, Argentina, have a future? Biol. Conserv. 120, 589–598.
Zarrilli A.G. (2008) El oro rojo. La industria del tanino en la Argentina (1890-1950). Silva Lusitana 16, 239–259.
Acknowledgments
The author thanks César Albornoz, Rogelio Burgardt and Mercedes Koler for their warm hospitality and help during the field studies in El Sauzalito and El Espinillo and Nora Brea and the three reviewers and editor for providing suggestions and comments which enrich the manuscript. I am especially grateful to Arturo Roig-Alsina for identifying the bees. This study was supported by CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas).
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript editor: Alexandra Klein
Evaluation de l'alimentation de pollen et d'association de fleurs de Melipona orbignyi et recommandations sur la gestion et la conservation des abeilles sans dard dans les forêts sèches du Chaco, en Amérique du Sud
Pollinisation vibratile / déforestation / pollinisation des forêts / méliponiculture / conservation du pollinisateur / pollinisateur de sous-étage
Beurteilung der Pollendiät und Blütenbeziehung bei Melipona orbignyi und Empfehlungen für Haltung und Schutz von stachellosen Bienen im Chaco Trockenwald von Südamerika.
Vibrationsbestäubung / Entwaldung / Waldbestäubung / Meliponinenimkerei / Bestäuberschutz / Unterholzbestäubung
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1.
(DOCX 22 kb)
Rights and permissions
About this article
Cite this article
Vossler, F.G. Pollen diet assessment and flower association in Melipona orbignyi and recommendations on management and conservation of stingless bees in the Chaco dry forest of South America. Apidologie 50, 391–413 (2019). https://doi.org/10.1007/s13592-019-00653-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13592-019-00653-4