Abstract
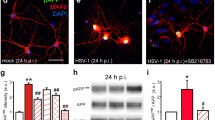
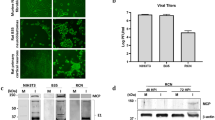
Several data indicate that neuronal infection with herpes simplex virus type 1 (HSV-1) causes biochemical alterations reminiscent of Alzheimer’s disease (AD) phenotype. They include accumulation of amyloid-β (Aβ), which originates from the cleavage of amyloid precursor protein (APP), and hyperphosphorylation of tau protein, which leads to neurofibrillary tangle deposition. HSV-1 infection triggers APP processing and drives the production of several fragments including APP intracellular domain (AICD) that exerts transactivating properties. Herein, we analyzed the production and intracellular localization of AICD following HSV-1 infection in neurons. We also checked whether AICD induced the transcription of two target genes, neprilysin (nep) and glycogen synthase kinase 3β (gsk3β), whose products play a role in Aβ clearance and tau phosphorylation, respectively. Our data indicate that HSV-1 led to the accumulation and nuclear translocation of AICD in neurons. Moreover, results from chromatin immunoprecipitation assay showed that AICD binds the promoter region of both nep and gsk3β. Time course analysis of NEP and GSK3β expression at both mRNA and protein levels demonstrated that they are differently modulated during infection. NEP expression and enzymatic activity were initially stimulated but, with the progression of infection, they were down-regulated. In contrast, GSK3β expression remained nearly unchanged, but the analysis of its phosphorylation suggests that it was inactivated only at later stages of HSV-1 infection. Thus, our data demonstrate that HSV-1 infection induces early upstream events in the cell that may eventually lead to Aβ deposition and tau hyperphosphorylation and further suggest HSV-1 as a possible risk factor for AD.





Similar content being viewed by others
References
Avrahami L, Farfara D, Shaham-Kol M, Vassar R, Frenkel D, Eldar-Finkelman H (2013) Inhibition of glycogen synthase kinase-3 ameliorates β-amyloid pathology and restores lysosomal acidification and mammalian target of rapamycin activity in the Alzheimer disease mouse model: in vivo and in vitro studies. J Biol Chem 288:1295–1306
Belyaev ND, Nalivaeva NN, Makova NZ, Turner AJ (2009) Neprilysin gene expression requires binding of the amyloid precursor protein intracellular domain to its promoter: implications for Alzheimer disease. EMBO Rep 10:94–100. doi:10.1038/embor.2008.222
Caccamo A, Oddo S, Sugarman MC, Akbari Y, LaFerla FM (2005) Age- and region-dependent alterations in Abeta-degrading enzymes: implications for Abeta-induced disorders. Neurobiol Aging 26:645–654
Cao X, Südhof TC (2001) A transcriptionally [correction of transcriptively] active complex of APP with Fe65 and histone acetyltransferase Tip60. Science 293:115–120
Chang KA, Kim HS, Ha TY, Ha JW, Shin KY, Jeong YH, Lee JP, Park CH, Kim S, Baik TK, Suh YH (2006) Phosphorylation of amyloid precursor protein (APP) at Thr668 regulates the nuclear translocation of the APP intracellular domain and induces neurodegeneration. Mol Cell Biol 26:4327–4338
De Chiara G, Marcocci ME, Civitelli L, Argnani R, Piacentini R, Ripoli C, Manservigi R, Grassi C, Garaci E, Palamara AT (2010) APP processing induced by herpes simplex virus type 1 (HSV-1) yields several APP fragments in human and rat neuronal cells. PLoS One 5, e13989. doi:10.1371/journal.pone.0013989
De Chiara G, Marcocci ME, Sgarbanti R, Civitelli L, Ripoli C, Piacentini R, Garaci E, Grassi C, Palamara AT (2012) Infectious agents and neurodegeneration. Mol Neurobiol 46:614–638
Dobson CB, Itzhaki RF (1999) Herpes simplex virus type 1 and Alzheimer’s disease. Neurobiol Aging 20:457–465
El-Amouri SS, Zhu H, Yu J, Marr R, Verma IM, Kindy MS (2008) Neprilysin: an enzyme candidate to slow the progression of Alzheimer’s disease. Am J Pathol 172:1342–1354
Fukami S, Watanabe K, Iwata N, Haraoka J, Lu B, Gerard NP, Gerard C, Fraser P, Westaway D, St George-Hyslop P, Saido TC (2002) Abeta-degrading endopeptidase, neprilysin, in mouse brain: synaptic and axonal localization inversely correlating with Abeta pathology. Neurosci Res 43:39–56
Goodger ZV, Rajendran L, Trutzel A, Kohli BM, Nitsch RM, Konietzko U (2009) Nuclear signaling by the APP intracellular domain occurs predominantly through the amyloidogenic processing pathway. J Cell Sci 122:3703–3714
Haass C, Kaether C, Thinakaran G, Sisodia S (2012) Trafficking and proteolytic processing of APP. Cold Spring Harb Perspect Med 5:a006270
Hooper C, Killick R, Lovestone S (2008) The GSK3 hypothesis of Alzheimer’s disease. J Neurochem 104:1433–1439
Iwata N, Tsubuki S, Takaki Y, Shirotani K, Lu B, Gerard NP, Gerard C, Hama E, Lee HJ, Saido TC (2001) Metabolic regulation of brain Abeta by neprilysin. Science 292:1550–1552
Kanemitsu H, Tomiyama T, Mori H (2003) Human neprilysin is capable of degrading amyloid beta peptide not only in the monomeric form but also the pathological oligomeric form. Neurosci Lett 350:113–116
Kavouras JH, Prandovszky E, Valyi-Nagy K, Kovacs SK, Tiwari V, Kovacs M, Shukla D, Valyi-Nagy T (2007) Herpes simplex virus type 1 infection induces oxidative stress and the release of bioactive lipid peroxidation by-products in mouse P19N neural cell cultures. J Neurovirol 13:416–425
Killington RA, Powell KL (1991) Growth, assay and purification of herpesviruses. In: Mahy BWJ (ed), IRL Press Virology: a pratical approach, Oxford. pp. 212–213
Kimberly WT, Zheng JB, Guénette SY, Selkoe DJ (2001) The intracellular domain of the β-amyloid precursor protein is stabilized by Fe65 and translocates to the nucleus in a notch-like manner. J Biol Chem 276:40288–40292
Lefranc-Jullien S, Sunyach C, Checler F (2006) APPε, the ε-secretase-derived N-terminal product of the β-amyloid precursor protein, behaves as a type I protein and undergoes α-, β-, and γ-secretase cleavages. J Neurochem 97:807–817
Lerchundi R, Neira R, Valdivia S, Vio K, Concha MI, Zambrano A, Otth C (2011) Tau cleavage at D421 by caspase-3 is induced in neurons and astrocytes infected with herpes simplex virus type 1. J Alzheimers Dis 23:513–520
Leroy K, Yilmaz Z, Brion JP (2007) Increased level of active GSK-3beta in Alzheimer’s disease and accumulation in argyrophilic grains and in neurones at different stages of neurofibrillary degeneration. Neuropathol Appl Neurobiol 33:43–55
Lesné S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH (2006) A specific amyloid-beta protein assembly in the brain impairs memory. Nature 440:352–357
Li C, Booze RM, Hersh LB (1995) Tissue-specific expression of rat neutral endopeptidase (neprilysin) mRNAs. J Biol Chem 270:5723–5728
Masters CL, Selkoe DJ (2012) Biochemistry of amyloid β-protein and amyloid deposits in Alzheimer disease. Cold Spring Harb Perspect Med 2:a006262
Medina M, Wandosell F (2011) Deconstructing GSK-3: the fine regulation of its activity. Int J Alzheimers Dis 2011:479249
Mori I, Kimura Y, Naiki H, Matsubara R, Takeuchi T et al (2004) Reactivation of HSV-1 in the brain of patients with familial Alzheimer’s disease. J Med Virol 73:605–611
Naghavi MH, Gundersen GG, Walsh D (2013) Plus-end tracking proteins, CLASPs, and a viral Akt mimic regulate herpesvirus-induced stable microtubule formation and virus spread. Proc Natl Acad Sci U S A 110:18268–18273. doi:10.1073/pnas.1310760110
Nixon RA (2007) Autophagy, amyloidogenesis and Alzheimer disease. J Cell Sci 120:4081–4091
Palamara AT, Perno CF, Ciriolo MR, Dini L, Balestra E, D’Agostini C, Di Francesco P, Favalli C, Rotilio G, Garaci E (1995) Evidence for antiviral activity of glutathione: in vitro inhibition of herpes simplex virus type 1 replication. Antiviral Res 27:237–353
Piacentini R, Civitelli L, Ripoli C, Marcocci ME, De Chiara G, Garaci E, Azzena GB, Palamara AT, Grassi C (2011) HSV-1 promotes Ca2+-mediated APP phosphorylation and Aβ accumulation in rat cortical neurons. Neurobiol Aging 32:2323.e13–2323.e26. doi:10.1016/j.neurobiolaging.2010.06.009
Piacentini R, De Chiara G, Li Puma DD, Ripoli C, Marcocci ME, Garaci E, Palamara AT, Grassi C (2014) HSV-1 and Alzheimer’s disease: more than a hypothesis. Front Pharmacol 5:97
Santana S, Recuero M, Bullido MJ, Valdivieso F, Aldudo J (2012) Herpes simplex virus type I induces the accumulation of intracellular β-amyloid in autophagic compartments and the inhibition of the non-amyloidogenic pathway in human neuroblastoma cells. Neurobiol Aging 33:430.e19–430.e33
Sergeant N, David JP, Champain D, Ghestem A, Wattez A, Delacourte A (2002) Progressive decrease of amyloid precursor protein carboxy terminal fragments (APP-CTFs), associated with tau pathology stages, in Alzheimer’s disease. J Neurochem 81:663–672
Shipley SJ, Parkin ET, Itzhaki RF, Dobson CB (2005) Herpes simplex virus interferes with amyloid precursor protein processing. BMC Microbiol 5:48
Słomnicki LP, Leśniak W (2008) A putative role of the amyloid precursor protein intracellular domain (AICD) in transcription. Acta Neurobiol Exp 68:219–228
von Rotz RC, Kohli BM, Bosset J, Meier M, Suzuki T, Nitsch RM, Konietzko U (2004) The APP intracellular domain forms nuclear multiprotein complexes and regulates the transcription of its own precursor. J Cell Sci 117:4435–4448
Wang R, Wang S, Malter JS, Wang DS (2009) Effects of HNE-modification induced by Aβ on neprilysin expression and activity in SH-SY5Y cells. J Neurochem 108:1072–1082
Wang S, Wang R, Chen L, Bennett DA, Dickson DW, Wang DS (2010) Expression and functional profiling of neprilysin, insulin-degrading enzyme, and endothelin-converting enzyme in prospectively studied elderly and Alzheimer’s brain. J Neurochem 115:47–57
Weinmann AS, Farnham PJ (2002) Identification of unknown target genes of human transcription factors using chromatin immunoprecipitation. Methods 26:37e47
Wozniak MA, Itzhaki RF, Shipley SJ, Dobson CB (2007) Herpes simplex virus infection causes cellular beta-amyloid accumulation and secretase upregulation. Neurosci Lett 429:95–100
Wozniak MA, Frost AL, Itzhaki RF (2009) Alzheimer’s disease-specific tau phosphorylation is induced by herpes simplex virus type 1. J Alzheimers Dis 16:341–350. doi:10.3233/JAD-2009-0963
Xu F, Sternberg M, Kottiri B, McQuillan G, Lee F, Nahmias AJ, Berman SM, Markowitz LE (2006) Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 296:964–973
Yang DS, Stavrides P, Mohan PS, Kaushik S, Kumar A, Ohno M, Schmidt SD, Wesson D, Bandyopadhyay U, Jiang Y, Pawlik M, Peterhoff CM, Yang AJ, Wilson DA, St George-Hyslop P, Westaway D, Mathews PM, Levy E, Cuervo AM, Nixon RA (2011) Reversal of autophagy dysfunction in the TgCRND8 mouse model of Alzheimer’s disease ameliorates amyloid pathologies and memory deficits. Brain 134:258–277
Zambrano A, Solis L, Salvadores N, Cortés M, Lerchundi R, Otth C (2008) Neuronal cytoskeletal dynamic modification and neurodegeneration induced by infection with herpes simplex virus type 1. J Alzheimers Dis 14:259–269
Zhang YW, Thompson R, Zhang H, Xu H (2011) APP processing in Alzheimer’s disease. Mol Brain 4:3
Zhou L, Wei C, Huang W, Bennett DA, Dickson DW, Wang R, Wang D (2013) Distinct subcellular patterns of neprilysin protein and activity in the brains of Alzheimer’s disease patients, transgenic mice and cultured human neuronal cells. Am J Transl Res 5:608–621
Acknowledgments
The authors acknowledge Dr. Katarina Kågedal for critically reading the manuscript and Dr. Salvatore Fusco for his valuable technical advices. This work was supported by grants from the Italian Ministry of Education University and Research (PRIN2009PM9B33 to Anna Teresa Palamara and Claudio Grassi and PON01- 01802 to Anna Teresa Palamara).
Conflict of interest
All authors, Livia Civitelli, Maria Elena Marcocci, Ignacio Celestino, Roberto Piacentini, Enrico Garaci, Claudio Grassi, Giovanna De Chiara and Anna Teresa Palamara, declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Livia Civitelli and Maria Elena Marcocci contributed equally to this work.
Rights and permissions
About this article
Cite this article
Civitelli, L., Marcocci, M.E., Celestino, I. et al. Herpes simplex virus type 1 infection in neurons leads to production and nuclear localization of APP intracellular domain (AICD): implications for Alzheimer’s disease pathogenesis. J. Neurovirol. 21, 480–490 (2015). https://doi.org/10.1007/s13365-015-0344-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-015-0344-0