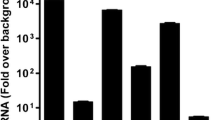
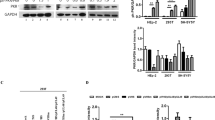
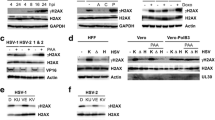
Abstract
Development of novel prevention and treatment strategies for herpes simplex virus (HSV) mediated diseases is dependent upon an accurate understanding of the central molecular events underlying the regulation of latency and reactivation. We have recently shown that the transactivation function of the virion protein VP16 is a critical determinant in the exit from latency in vivo. HSV-1 strain SJO2 carries a single serine to alanine substitution at position 375 in VP16 which disrupts its interaction with its essential co-activator Oct-1. Here we report that SJO2 is severely impaired in its ability to exit latency in vivo. This result reinforces our prior observations with VP16 transactivation mutant, in1814, in which VP16 interaction with Oct-1 is also disrupted and solidifies the importance of the VP16–Oct-1 interaction in the early steps in HSV-1 reactivation.


Similar content being viewed by others
References
Ace CI, McKee TA et al (1989) Construction and characterization of a herpes simplex virus type 1 mutant unable to transinduce immediate-early gene expression. J Virol 63(5):2260–2269
Ecob-Prince MS, Preston CM et al (1993a) Neurons containing latency-associated transcripts are numerous and widespread in dorsal root ganglia following footpad inoculation of mice with herpes simplex virus type 1 mutant in1814. J Gen Virol 74(Pt 6):985–994
Ecob-Prince MS, Rixon FJ et al (1993b) Reactivation in vivo and in vitro of herpes simplex virus from mouse dorsal root ganglia which contain different levels of latency-associated transcripts. J Gen Virol 74(Pt 6):995–1002
Glynn JR, Biraro S et al (2009) Herpes simplex virus type 2: a key role in HIV incidence. AIDS 23(12):1595–1598
Harris RA, Preston CM (1991) Establishment of latency in vitro by the herpes simplex virus type 1 mutant in1814. J Gen Virol 72(Pt 4):907–913
Honess RW, Roizman B (1974) Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol 14(1):8–19
Itzhaki RF, Wozniak MA (2008) Herpes simplex virus type 1 in Alzheimer's disease: the enemy within. J Alzheimers Dis 13(4):393–405
Knipe D (2007) Fields virology. Lippincott Williams & Wilkins, Philadelphia
Lam Q, Smibert CA et al (1996) Herpes simplex virus VP16 rescues viral mRNA from destruction by the virion host shutoff function. EMBO J 15(10):2575–2581
McFarlane M, Daksis JI et al (1992) Hexamethylene bisacetamide stimulates herpes simplex virus immediate early gene expression in the absence of trans-induction by Vmw65. J Gen Virol 73(Pt 2):285–292
Mossman KL, Sherburne R et al (2000) Evidence that herpes simplex virus VP16 is required for viral egress downstream of the initial envelopment event. J Virol 74(14):6287–6299
Mukamal KJ, Kronmal RA et al (2004) Traditional and novel risk factors in older adults: cardiovascular risk assessment late in life. Am J Geriatr Cardiol 13(2):69–80
O'Reilly D, Hanscombe O et al (1997) A single serine residue at position 375 of VP16 is critical for complex assembly with Oct-1 and HCF and is a target of phosphorylation by casein kinase II. EMBO J 16(9):2420–2430
Ottosen S, Herrera FJ et al (2006) Phosphorylation of the VP16 transcriptional activator protein during herpes simplex virus infection and mutational analysis of putative phosphorylation sites. Virology 345(2):468–481
Pepose JS, Keadle TL et al (2006) Ocular herpes simplex: changing epidemiology, emerging disease patterns, and the potential of vaccine prevention and therapy. Am J Ophthalmol 141(3):547–557
Preston CM, McFarlane M (1998) Cytodifferentiating agents affect the replication of herpes simplex virus type 1 in the absence of functional VP16. Virology 249(2):418–426
Proenca JT, HM Coleman et al. (2011) An investigation of HSV promoter activity compatible with latency establishment reveals VP16 independent activation of HSV immediate early promoters in sensory neurones. J Gen Virol 92(11):2575–2585
Roberts S (2009) Herpes simplex virus: incidence of neonatal herpes simplex virus, maternal screening, management during pregnancy, and HIV. Curr Opin Obstet Gynecol 21(2):124–130
Roizman B, Gu H et al (2005) The first 30 minutes in the life of a virus: unREST in the nucleus. Cell Cycle 4(8):1019–1021
Sawtell NM (1997) Comprehensive quantification of herpes simplex virus latency at the single-cell level. J Virol 71(7):5423–5431
Sawtell NM (1998) The probability of in vivo reactivation of herpes simplex virus type 1 increases with the number of latently infected neurons in the ganglia. J Virol 72(8):6888–6892
Sawtell NM (2003) Quantitative analysis of herpes simplex virus reactivation in vivo demonstrates that reactivation in the nervous system is not inhibited at early times postinoculation. J Virol 77(7):4127–4138
Sawtell NM (2005) Detection and quantification of the rare latently infected cell undergoing herpes simplex virus transcriptional activation in the nervous system in vivo. Methods Mol Biol 292:57–72
Sawtell NM, Thompson RL (1992) Herpes simplex virus type 1 latency-associated transcription unit promotes anatomical site-dependent establishment and reactivation from latency. J Virol 66(4):2157–2169
Sawtell NM, Thompson RL et al (2006) Herpes simplex virus DNA synthesis is not a decisive regulatory event in the initiation of lytic viral protein expression in neurons in vivo during primary infection or reactivation from latency. J Virol 80(1):38–50
Sears AE, Hukkanen V et al (1991) Expression of the herpes simplex virus 1 alpha transinducing factor (VP16) does not induce reactivation of latent virus or prevent the establishment of latency in mice. J Virol 65(6):2929–2935
Smiley JR, Duncan J (1997) Truncation of the C-terminal acidic transcriptional activation domain of herpes simplex virus VP16 produces a phenotype similar to that of the in1814 linker insertion mutation. J Virol 71(8):6191–6193
Steiner I, Spivack JG et al (1990) A herpes simplex virus type 1 mutant containing a nontransinducing Vmw65 protein establishes latent infection in vivo in the absence of viral replication and reactivates efficiently from explanted trigeminal ganglia. J Virol 64(4):1630–1638
Stone MJ, Hawkins CP (2007) A medical overview of encephalitis. Neuropsychol Rehabil 17(4–5):429–449
Sun Y, Pei W et al (2005) An association of herpes simplex virus type 1 infection with type 2 diabetes. Diabetes Care 28(2):435–436
Tal-Singer R, Pichyangkura R et al (1999) The transcriptional activation domain of VP16 is required for efficient infection and establishment of latency by HSV-1 in the murine peripheral and central nervous systems. Virology 259(1):20–33
Thompson RL, Preston CM et al (2009) De novo synthesis of VP16 coordinates the exit from HSV latency in vivo. PLoS Pathog 5(3):e1000352
Thompson RL, Sawtell NM (2006) Evidence that the herpes simplex virus type 1 ICP0 protein does not initiate reactivation from latency in vivo. J Virol 80(22):10919–10930
Valyi-Nagy T, Deshmane SL et al (1991) Investigation of herpes simplex virus type 1 (HSV-1) gene expression and DNA synthesis during the establishment of latent infection by an HSV-1 mutant, in1814, that does not replicate in mouse trigeminal ganglia. J Gen Virol 72(Pt 3):641–649
Visser, M. R. and G. M. Vercellotti (1993) Herpes simplex virus and atherosclerosis. Eur Heart J 14 Suppl K: 39–42.
Wysocka J, Herr W (2003) The herpes simplex virus VP16-induced complex: the makings of a regulatory switch. Trends Biochem Sci 28(6):294–304
Acknowledgements
This work was supported by Public Health Service NIH grants AI32121 to NMS and EY13168 to RLT.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sawtell, N.M., Triezenberg, S.J. & Thompson, R.L. VP16 serine 375 is a critical determinant of herpes simplex virus exit from latency in vivo. J. Neurovirol. 17, 546–551 (2011). https://doi.org/10.1007/s13365-011-0065-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-011-0065-y