Abstract
Introduction
Liraglutide is a glucagon-like peptide-1 analogue used to treat type 2 diabetes mellitus (T2DM). To date, limited long-term data (> 2 years) exist comparing real-world diabetes-related effectiveness and costs for liraglutide versus insulin treatment.
Methods
This retrospective claims data analysis covered the period from 1 January 2010 to 31 December 2017 and included continuously insured patients with T2DM who initiated insulin or liraglutide and had 3.5 or 5 years’ follow-up data, identified using the German AOK PLUS dataset. Propensity score matching (PSM) was used to adjust for patient characteristics.
Results
After PSM, there were 825 and 436 patients in the liraglutide and insulin groups at 3.5 and 5 years’ follow-up, respectively. Baseline characteristics were similar between compared cohorts. The respective change from baseline to follow-up in mean glycated haemoglobin for liraglutide and insulin patients was − 0.88% and − 0.81% (p > 0.100) after 3.5 years and − 1.15%/ − 1.02% (p > 0.100) after 5 years. Mean respective changes in body mass index (kg/m2) were − 1.21/+ 1.14 (p < 0.001) after 3.5 years and − 1.29/+ 1.13 after 5 years (p < 0.001). Liraglutide- versus insulin-treated patients were less likely to have an early T2DM-related hospitalisation (3.5-year hazard ratio [HR]: 0.414 [95% confidence interval (CI) 0.263–0.651]; 5-year HR: 0.448 [95% CI 0.286–0.701]). At 5 years’ follow-up, there was no statistically significant difference in total direct costs between treatment groups (cost ratio: 1.069 [95% CI 0.98–1.13]; p > 0.100).
Conclusion
The clinical effectiveness of liraglutide is maintained long term (up to 5 years). Liraglutide treatment is not associated with higher total direct healthcare costs.
Similar content being viewed by others
Why carry out this study? |
To date, limited long-term (> 2 years) real-world evidence exists regarding effectiveness and diabetes-related costs associated with liraglutide versus insulin treatment in patients with type 2 diabetes mellitus. |
Most studies conducted to date have been performed outside of Europe. |
This study investigated long-term (up to 5 years) diabetes-related costs and effectiveness of liraglutide and insulin in German patients who were naive to either treatment prior to initiation. |
What was learned from the study? |
The study showed that clinical effectiveness achieved with liraglutide treatment was maintained for up to 5 years. |
Body mass index, systolic blood pressure and glycated haemoglobin levels improved with liraglutide treatment compared with insulin. |
Liraglutide treatment was associated with lower healthcare costs (outpatient, inpatient and medical aids), but with higher drug costs; at 5 years’ follow-up, there was no statistically significant difference in total costs between treatment groups. |
Introduction
The global prevalence of type 2 diabetes mellitus (T2DM) is rising [1, 2] and has quadrupled in the last 3 decades [3]. In Germany, the current prevalence of diabetes is estimated to be between 7.2 and 9.9% [4]. It is projected that between 2015 and 2040, there will be a 21% increase in the prevalence of T2DM in Germany [5]. T2DM poses a significant disease burden, and in 2010 it was estimated that around 140,000 deaths in Germany were due to T2DM-related increased mortality [6]. Management of hyperglycaemia in T2DM focuses on lifestyle interventions such as diet and exercise, followed by pharmacological therapy, with metformin often being the first choice [7]. If this is insufficient, intensification with a second glucose-lowering drug is recommended [7,8,9,10,11]. Several second-line treatments are available, with different mechanisms of action, including sulphonylureas, dipeptidyl peptidase-4 (DPP-4) inhibitors, sodium-glucose co-transporter-2 (SGLT2) inhibitors and glucagon-like peptide-1 (GLP-1) analogues [7,8,9,10,11]. Until recently, basal insulin was also recommended as a second-line option in international guidelines [10, 11].
Liraglutide is a GLP-1 analogue that stimulates insulin secretion in a glucose-dependent manner [12]. Liraglutide is indicated for second-line treatment of patients with T2DM in combination with metformin and other oral antidiabetic drugs and/or insulin or as monotherapy if metformin cannot be tolerated or is contraindicated [13]. In phase 3 clinical trials in adult patients with T2DM, liraglutide (as adjuvant treatment and monotherapy) improved glycaemic control, reduced body weight and/or lowered the risk of hypoglycaemia versus a range of comparators [14,15,16,17,18,19,20,21,22,23]. In some trials, liraglutide also lowered systolic blood pressure (SBP) [17, 18, 20, 22, 23]. In the LIBERTY non-interventional study conducted in Germany between 14 November 2011 and 31 October 2014, liraglutide treatment for 1 year improved certain Audit of Diabetes-Dependent Quality of Life components and reduced body mass index (BMI), SBP and non-severe hypoglycaemic episodes versus any insulin. Both treatments improved glycaemic control [24]. In patients with T2DM and at high risk of CV events in the LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) trial, liraglutide reduced the risk of major adverse CV events (a composite of CV death, non-fatal myocardial infarction and non-fatal stroke) compared with placebo as add-on to standard of care [25].
Given that insulin and liraglutide are both treatment options for T2DM, it is important to understand their long-term real-world outcomes. The real-world effectiveness of liraglutide has been previously investigated; however, despite the major importance of long-term effects on clinical outcomes in T2DM, limited data beyond 2 years are available and fewer still with insulin as a comparator [26]. This claims data analysis in adult patients with T2DM investigated diabetes-related effectiveness and costs with long-term (up to 5 years) treatment with liraglutide or any insulin in a real-world setting.
Methods
Study Design
A retrospective study was conducted using administrative claims from a German health fund (AOK PLUS), located in the Saxony/Thuringia region of Germany. The fund insures > 3.2 million people and gives access to the data of > 400,000 patients with T2DM. Data used for the analysis covered the period from 1 January 2010 to 31 December 2017 and included available data for patients with T2DM (International Statistical Classification of Diseases and Related Health Problems [ICD]-10 code: E11) documented in a disease management programme (DMP). The study included continuously insured patients with T2DM who initiated insulin or liraglutide, continued that therapy for 3.5 or 5 years and had (except for death) follow-up data available at 3.5 or 5 years. Baseline data were collected within 6 months prior to initiation of liraglutide or insulin.
Ethical Considerations
Ethics approval was granted for the preceding LIBERTY study, which included approval for long-term (up to 5 years) data analysis of participating patients who had AOK PLUS health fund data available. Not all patients in the current study took part in the LIBERTY study. However, as the evaluation was based upon retrospective analysis of anonymised data from a health fund, it was not considered necessary to gain ethics approval or seek patient consent for the additional patients included in this analysis.
Patients and Data
Inclusion Criteria
Patients had T2DM and were naive to both liraglutide (Anatomical Therapeutic Chemical [ATC] code A10BX07/A10BJ02) and insulin (ATC code A10A*) prior to inclusion, defined as no prescription of liraglutide or any insulin within 6 months prior to indexing. Patients could have prior history of other GLP-1 analogue use or any other T2DM-related medication. Patients were initiated on treatment with liraglutide (≤ 1.8 mg once daily) or any type of insulin (human insulin or insulin analogues, in any device and in any insulin treatment regimen such as basal, pre-mixed or basal-bolus therapy).
Analysis Sets
The primary analysis sets for the 3.5- and 5-year follow-up groups were made up of two different datasets:
-
1.
Patients naive to both insulin and liraglutide who started liraglutide and remained on liraglutide treatment during the follow-up period.
-
2.
Patients naive to both insulin and liraglutide who started insulin and remained on insulin treatment during the follow-up period.
In case of death of a patient, censoring was performed. In addition to the above analysis, a sensitivity analysis compared subsamples of patients who were treated for the duration of the observational period without the respective competitor being used as an add-on. Results for the sensitivity analysis are given here descriptively only, but the data are available on request from the corresponding author.
Propensity Score Matching
When comparing individuals receiving different treatments in non-randomised groups, the question of comparability due to systematic differences across treatment groups needs to be addressed. Propensity score matching (PSM) is a common approach to adjust for patients’ characteristics [27]. Patients with the same propensity score have theoretically the same probabilistic distribution over other covariates, independently of the treatment they received. Propensity scores were estimated using logistic regression models, where the dependent variable was a binary indicator of group affiliation (liraglutide versus insulin). All available characteristics of each patient in the 6 months prior to indexing (before either liraglutide or any insulin was initiated), such as demographics and comorbid conditions, were used as potential independent predictor variables. Those variables found to be significant with backward stepwise regression, as well as parameters with obvious clinical relevance regarding treatment decision (irrespective of significance), were used to calculate the propensity scores. Therefore, the following available variables were included: age in years; gender; index year (year of treatment initiation); prescribed antidiabetic drugs in the 12-month pre-index period; number of and top ten of other medications prescribed in the 12-month pre-index period; Charlson Comorbidity Index based on respective diagnoses in the 12-month pre-index period; adapted Diabetes Complications Severity Index based on respective diagnoses in the 12-month pre-index period; top ten documented diagnosis in the 12-month pre-index period; diabetes-related complications in the 12-month pre-index period; number of hospitalisations with ‘diabetes’ as the main diagnosis in the 12-month pre-index period; number of hospitalisations with ‘other’ as the main diagnosis in the 12-month pre-index period; number of general practitioner (GP) visits in the 12-month pre-index period; number of outpatient specialist visits in the 12-month pre-index period; baseline HbA1c; baseline BMI; baseline SBP.
Based on the estimated propensity scores, patients from the two groups (liraglutide versus insulin) were matched using a nearest-neighbour 1:1 matching algorithm with a maximum caliper of 0.001.
Treatments Administered
Patients initiated treatment with either liraglutide or any insulin, as prescribed by their treating physician (see “Inclusion Criteria” for further information). There were no restrictions around the use of other concomitant medications including GLP-1 analogues and other glucose-lowering drugs.
Study Endpoints
The aims of the study were to investigate differences in the development of clinical parameters (HbA1c, BMI and SBP), diabetes-related complications and long-term diabetes-related costs. Time to first diabetes-related hospitalisation (diabetes as primary diagnosis, which also captured acute hypoglycaemic events) was also recorded. Early T2DM-related hospitalisation was defined as hospitalisation between the index date and 3.5 or 5 years.
Diabetes-related concomitant medications were coded and reported based on ATC classification standards and included metformin, sulphonylureas, combinations of oral blood glucose-lowering drugs, alpha-glucosidase inhibitors, thiazolidinediones, DPP-4 inhibitors, GLP-1 receptor agonists, SGLT2 inhibitors, other blood glucose-lowering drugs [excluding insulin], liraglutide/insulin with any other antidiabetic drug and liraglutide/insulin without any other antidiabetic drug.
Patients had at least BMI and HbA1c values available at baseline (measured in the 6 months pre-index using the value measured closest to the index date). Follow-up measures considered clinical parameters measured within ± 30 days of the respective follow-up date, using the value measured closest to the follow-up date. As such, baseline values were complete for all patients; however, there were a decreasing number of patients during the follow-up periods due to missing data.
Diabetes-related complications investigated included hospitalisations due to T2DM, CV disease, cerebrovascular disease, nephropathy, amputation, peripheral vascular disease, neuropathy and vitrectomy (see Table S1 for a full list of applicable ICD-10 codes).
Diabetes-related costs were reported per observed patient year and included:
-
Medication costs, which considered all prescriptions of antidiabetic agents, i.e. ATC code A10*; assessed based on pharmacies’ selling price.
-
Outpatient costs, which considered all outpatient visits related to T2DM diagnosis and/or T2DM-related complications.
-
Inpatient costs, which considered all hospitalisations associated with T2DM diagnosis and/or T2DM-related complications as the main diagnosis; cost assessment was based on respective diagnosis-related groups.
-
Medical aids costs, which related to prescriptions with ATC V04CA and other invoiced aids (test strips, lancets, needles, education).
Statistical Analysis
Continuous variables were analysed descriptively. Mean and standard deviations were reported as the main summary measures, and medians were reported when the distribution was skewed. Frequency and percentages were used for all categorical variables. The 95% confidence interval (CI) was displayed in the longitudinal comparisons of continuous variables. All CIs, statistical tests and resulting p values were two-sided. All hypothesis testing was performed at the 5% significance level. Analysis of time to first diabetes-related hospitalisation was based on Kaplan-Meier analysis models and depicted the percentage of patients still event-free since treatment initiation. For all outcome measures, data were collected for up to 5 years after treatment initiation. No imputation of missing data was performed.
Results
Study Population
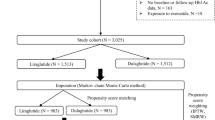
The numbers of patients identified for inclusion before and after PSM are presented in Fig. 1. Demographics and baseline characteristics were similar between the two treatment groups after PSM at both 3.5- and 5-year follow-up and are presented in Table 1. Due to censoring in case of death of a patient, the average follow-up times for the 3.5- and 5-year follow-up cohorts were 3.45 and 4.89 years, respectively, for the liraglutide group and 3.36 and 4.76 years, respectively, for the insulin group.
At both 3.5- and 5-year follow-up, more patients in the liraglutide group received additional insulin during the follow-up period compared with patients in the insulin group who received additional liraglutide during the follow-up period (Table 1).
Concomitant T2DM-related medications taken by patients at 3.5- and 5-year follow-up for primary analysis are presented in Table S2. More patients in the liraglutide versus insulin group took concomitant metformin and sulphonylureas during the follow-up period. More patients in the insulin versus liraglutide group took concomitant DPP-4 inhibitors during the follow-up period.
Clinical Effectiveness
HbA1c
The results for mean changes from baseline in HbA1c in the 3.5- and 5-year follow-up cohorts are presented in Fig. 2. With both liraglutide and insulin treatment, mean improvements (reductions) from baseline were seen in HbA1c, with a tendency towards greater reductions from baseline in patients who initiated liraglutide versus insulin treatment. In the liraglutide-treatment groups, HbA1c reductions happened earlier after initiation than in those treated with insulin (Fig. 2). Differences between treatment groups were statistically significant in favour of liraglutide at 6 (p < 0.001), 12 (p < 0.05) and 18 (p < 0.01) months in the 3.5-year follow-up cohort and at 12 (p < 0.05) and 24 (p < 0.05) months in the 5-year follow-up cohort.
Mean change from baseline in HbA1c by time at i 3.5-year follow-up and ii 5-year follow-up. Statistical comparisons were made between liraglutide and insulin at each time point. All testing was performed at the 5% significance level, where *p < 0.05; **p < 0.01; ***p < 0.001. Note: Patient numbers detail the number of patients with available information/data at each time point but may not be the same patients at each time point. HbA1c, glycated haemoglobin
BMI
The results for mean changes from baseline in BMI in the 3.5- and 5-year follow-up cohorts are presented in Fig. 3. Mean improvements (reductions) from baseline in BMI values were observed in the liraglutide groups only. Differences between the liraglutide and insulin groups were statistically significant in favour of liraglutide treatment at all follow-up time points in both the 3.5- and 5-year follow-up cohorts (p < 0.001). In patients who initiated insulin treatment, increases in mean BMI from baseline were seen at all follow-up time points.
Mean change from baseline in BMI by time at i 3.5-year follow-up and ii 5-year follow-up. Statistical comparisons were made between liraglutide and insulin at each time point. All testing was performed at the 5% significance level, where ***p < 0.001. Note: Patient numbers detail the number of patients with available information/data at each time point but may not be the same patients at each time point. BMI, body mass index
SBP
The results for mean SBP change over time in the 3.5- and 5-year follow-up cohorts are presented in Fig. 4. Mean SBP values were below baseline at all follow-up time points for patients in the liraglutide groups. There was no discernible pattern for patients in the insulin groups. Statistically significant differences (p < 0.05 − p < 0.01) in favour of liraglutide were seen in the 3.5-year follow-up cohort only at all but the 18-month time point.
Mean systolic blood pressure by time at i 3.5-year follow-up and ii 5-year follow-up. Statistical comparisons were made between liraglutide and insulin at each time point. All testing was performed at the 5% significance level, where *p < 0.05; **p < 0.01. Note: Patient numbers detail the number of patients with available information/data at each time point but may not be the same patients at each time point. BL, baseline
Diabetes-Related Complications
Kaplan-Meier comparisons of time to first T2DM-related hospitalisation (which included acute hypoglycaemic events) after treatment initiation are presented for the primary analysis set in Fig. 5. Patients treated with liraglutide were less likely to have an early T2DM-related hospitalisation than patients treated with insulin (3.5-year follow-up hazard ratio [HR]: 0.414 [95% CI 0.263–0.651]; 5-year follow-up HR: 0.448 [95% CI 0.286–0.701]).
Results for hospitalisations due to T2DM-related disorders or complications in the 3.5- and 5-year follow-up cohorts are presented in Table S1. Overall, liraglutide-treated patients had a significantly lower risk of T2DM-related complications versus insulin-treated patients. Rate ratios (RRs) of the general incidence of T2DM-related complications comparing liraglutide versus insulin treatment were all < 0.500. RRs for specific T2DM-related complications were in favour of liraglutide for CV events, amputation, nephropathy and vitrectomy.
Diabetes-Related Costs
Results for diabetes-related costs at 3.5- and 5-year follow-up for the primary analysis set are presented in Fig. 6. Inpatient and medical aid costs were significantly lower for patients who initiated liraglutide versus insulin treatment at both 3.5 and 5 years of follow-up. Outpatient costs were similar at both 3.5- and 5-year follow-up. T2DM-related medication costs were significantly higher for patients who initiated liraglutide versus insulin treatment at both 3.5- and 5-year follow-up. At 3.5 years’ follow-up, total costs were lower for patients who initiated insulin treatment. The cost RR was 1.066 and was statistically significant (95% CI 1.01–1.12; p = 0.016). At 5 years’ follow-up, there was no statistically significant difference in total costs between treatment groups. The cost ratio was 1.069 (95% CI 0.98–1.13; p > 0.100).
Results of Sensitivity Analysis
Sensitivity analysis results in a subsample of patients who were treated without the respective competitor as an add-on for the duration of the observational period were mostly in accordance with the primary analysis set. One difference was in respect to diabetes-related costs. In contrast to the primary analysis set, in the sensitivity analysis the cost difference reversed to favour liraglutide at 3.5 years’ follow-up, although the difference was not statistically significant. The cost ratio was 0.989 (95% CI 0.93–1.05; p > 0.100).
Discussion
To date, there has been a lack of long-term (> 2 years’ follow-up) T2DM-related cost and clinical effectiveness data for patients newly initiating liraglutide versus insulin therapy, especially in Europe. Therefore, the main objective of this study was to collect these data for patients treated in routine German clinical practice, using PSM to offset confounding.
In terms of clinical effectiveness, both liraglutide and insulin treatment were associated with mean improvements (reductions) from baseline in HbA1c. There was a tendency towards earlier and greater mean reductions from baseline in patients who initiated liraglutide versus insulin treatment; differences were statistically significant in favour of liraglutide up to 2 years in the 5-year follow-up cohort. Apparent convergence of control in HbA1c levels was seen with long-term (> 2 years) insulin and liraglutide treatment, possibly consistent with the insulin group intensifying insulin treatment with time. In the LIBERTY study, which included patients with T2DM from the same region of Germany who were insured by AOK PLUS and initiated liraglutide or insulin treatment, HbA1c reductions were greater in the insulin versus liraglutide treatment group at 1 year (− 2.0% versus − 1.2%; p < 0.01); however, mean HbA1c after 1 year was 7.2% in both groups [24]. One potential reason for the disparity between the LIBERTY study results and those reported here is that, in the LIBERTY study, patients with higher baseline HbA1c levels were more likely to initiate insulin than liraglutide [24], whereas in the current study, PSM was able to offset this potential confounding factor. The mean HbA1c reductions observed with liraglutide in this study were similar to those observed with liraglutide (1.2 mg or 1.8 mg once daily) in the phase 3 LEAD trials (0.6–1.5% points) [14,15,16,17,18,19, 28] and with studies conducted in other real-world settings (1.0–1.5% points) [29,30,31]. Our data show that liraglutide and insulin are both effective long-term treatment options for improving glycaemic control in patients with T2DM.
Consistent with findings from phase 3 clinical trials [14,15,16,17,18,19,20,21,22,23, 25], the LIBERTY study [23] and other observational studies conducted in Europe [29,30,31], liraglutide significantly decreased BMI versus insulin, an effect sustained in the 5-year follow-up cohort in the current study. Differences in BMI between liraglutide and insulin groups were statistically significant in favour of liraglutide at all follow-up time points (p < 0.001). Meanwhile, insulin treatment was associated with increases in mean BMI at all follow-up time points (up to 5 years). This apparent divergence of control in BMI over time with liraglutide versus insulin treatment is possibly consistent with the insulin group increasing their insulin dose with time.
In line with the LIBERTY study [24], there appeared to be a neutral effect of insulin on SBP. Reductions in mean SBP with liraglutide were observed in the 3.5-year cohort and were statistically significant versus insulin at all but the 18-month follow-up time point. In the 5-year follow-up cohort, in the liraglutide group, mean SBP decreased from baseline at all follow-up time points; however, a similar effect was noted in the insulin group, and there were no statistically significant differences between treatment groups. It should be noted that the current study did not control for the addition of different antihypertensive medicines, and the clinical relevance of the maximum (4 mmHg) reduction in mean SBP achieved with liraglutide remains to be determined. However, a relationship between elevated SBP and the incidence of CV events in patients with T2DM has been established [32]. Therefore, any reduction in SBP is likely to contribute to a reduction in the risk of CV complications in this patient population.
In terms of hospitalisations due to diabetes-related complications, in this claims database study, liraglutide-treated patients had a significantly lower risk of hospitalisations versus insulin-treated patients (3.5-year follow-up HR: 0.414 [95% CI 0.263–0.651]; 5-year follow-up HR: 0.448 [95% CI 0.286–0.701]). Positive effects of liraglutide on CV outcomes in patients with T2DM at high risk of CV events have been previously reported. In the LEADER trial, liraglutide added to standard of care (compared with placebo) reduced the risk of major adverse CV events including CV death, non-fatal myocardial infarction and non-fatal stroke [25].
Limited evidence has been published regarding the long-term costs of liraglutide versus insulin. Studies to date have been up to 1 year in duration and conducted in the US [33,34,35], where significant differences in the health system exist compared with Germany. Similarly, most published studies of other GLP-1 receptor agonists versus insulin were mainly conducted in the US and lasted up to 1 or 2 years [36,37,38,39,40]. The use of short-term studies to assess the value of an intervention designed to impact disease progression over intervals of years is of limited utility.
In the current analysis, at 5 years’ follow-up, there was no statistically significant difference in total costs between the liraglutide and insulin treatment groups. At 3.5 years’ follow-up, the higher T2DM-related medication cost observed in the liraglutide group compared with the insulin group could not be totally offset by the significantly lower inpatient and medical aid costs in the liraglutide group. However, in the sensitivity analysis, the total costs for insulin were non-significantly higher at 3.5 years’ follow-up. This could be due to there being fewer hospitalisation costs for events such as hypoglycaemia in the liraglutide group after the exclusion of patients also taking insulin.
Study Limitations
Observational studies are vulnerable to bias and confounding, especially in terms of participant selection, differences in data capture and reporting over the long-term duration. The current claims data study was designed to minimise selection bias, implemented quality assurance steps for data collection and adopted an appropriate analysis. A potential limitation is that the patients were all within a defined region of Germany and insured by one healthcare provider, and this could limit the generalisability of the findings to other regions or settings. Moreover, costs were based on list prices, and no specific rebates were taken into account. A further limitation is that this study did not differentiate between insulin regimens during follow-up or account for the varying costs between different insulins in the cost analysis. Use of concomitant medications during follow-up was not considered in the analyses. However, as this study investigated diabetes-related effectiveness in a real-world setting, the use of concomitant medications in the two groups will likely reflect the use of these medications in routine clinical practice and will, therefore, make the results of this study more relevant and applicable to a real-world setting. The PSM included a comprehensive suite of variables to ensure that patients were well matched based on baseline health status and other factors likely to confound outcomes. Variables such as socioeconomic status, marital status, educational attainment and smoking were not included in the PSM, however, and it is possible that these factors could have had a confounding effect. Additionally, the study sample size was relatively small and the mean BMI in the overall study population was 35.9 kg/m2, which is relatively high compared with that in other European studies in patients with T2DM. In two retrospective claims data analyses of 284,878 and 228,703 German patients, mean BMI was 30.6 kg/m2 and 30.2 kg/m2, respectively [41, 42]. In an observational study in Belgium, mean BMI was 33.9 kg/m2 [30]. This could limit the generalisability of these findings. However, in an observational study carried out in Spain in patients with T2DM, the mean BMI was higher than in this study, at 38.6 kg/m2 [31]. The high BMI of patients in this study likely reflects that liraglutide was being chosen for more overweight patients and that the PSM would then select the most overweight insulin-treated patients to achieve pairings.
Conclusion
This study demonstrates that the clinical effectiveness of liraglutide is maintained long term (up to 5 years), and clinical outcomes were better with liraglutide than with insulin treatment. Liraglutide treatment was associated with lower healthcare costs, but higher drug costs. At 5 years of follow-up, there was no statistically significant difference in total costs between treatment groups, and sensitivity analysis of the 3.5-year cohort gave the same result. These findings suggest that liraglutide is a suitable option for intensifying T2DM treatment following one or more oral antidiabetic drugs and is effective long term.
References
Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care. 2011;34:1249–57.
Papier K, Jordan S, Dste C, et al. Incidence and risk factors for type 2 diabetes mellitus in transitional Thailand: results from the Thai cohort study. BMJ Open. 2016;6:e014102.
Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88–98.
Heidemann C, Scheidt-Nave C. Prevalence, incidence and mortality of diabetes mellitus in adults in Germany – a review in the framework of the Diabetes Surveillance. J Health Monit. 2017;2:98–121.
Tönnies T, Röckl S, Hoyer A, et al. Projected number of people with diagnosed Type 2 diabetes in Germany in 2040. Diabetes Med. 2019;36:1217–25.
Jacobs E, Hoyer A, Brinks R, Kuss O, Rathmann W. Burden of mortality attributable to diagnosed diabetes: a nationwide analysis based on claims data from 65 million people in Germany. Diabetes Care. 2017;40:1703–9.
Pfeiffer AF, Klein HH. The treatment of type 2 diabetes. Dtsch Arztebl Int. 2014;111:69–81.
Amanda S, Butcher R. Sodium-glucose co-transporter 2 inhibitors for the treatment of type 2 diabetes: a review of clinical effectiveness, cost-effectiveness, and guidelines. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2019. https://www.ncbi.nlm.nih.gov/books/NBK545104/. Accessed 06 Nov 2019.
DDG: Deutsche diabetes gesellschaft. National guidelines. https://www.deutsche-diabetes-gesellschaft.de/leitlinien/evidenzbasierte-leitlinien.html. Accessed Dec 2019.
Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;2018(41):2669–701.
Buse J, Wexler D, Tsapas A, et al. 2019 Update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43:487–93.
Vanderheiden A, Harrison LB, Warshauer JT, et al. Mechanisms of action of liraglutide in patients with type 2 diabetes treated with high-dose insulin. J Clin Endocrinol Metab. 2016;101:1798–806.
Victoza summary of product characteristics. February 2018. https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001026/WC500050017.pdf. Accessed 06 Nov 2019.
Marre M, Shaw J, Brändle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabetes Med. 2009;26:268–78.
Nauck M, Frid A, Hermansen K, et al. Long-term efficacy and safety comparison of liraglutide, glimepiride and placebo, all in combination with metformin in type 2 diabetes: 2-year results from the LEAD-2 study. Diabetes Obes Metab. 2013;15:204–12.
Garber A, Henry RR, Ratner R, et al. Liraglutide, a once-daily human glucagon-like peptide 1 analogue, provides sustained improvements in glycaemic control and weight for 2 years as monotherapy compared with glimepiride in patients with type 2 diabetes. Diabetes Obes Metab. 2011;13:348–56.
Zinman B, Gerich J, Buse JB, et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met + TZD). Diabetes Care. 2009;32:1224–300.
Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met + SU): a randomised controlled trial. Diabetologia. 2009;52:2046–55.
Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374:39–47.
Pratley R, Nauck M, Bailey T, et al. One year of liraglutide treatment offers sustained and more effective glycaemic control and weight reduction compared with sitagliptin, both in combination with metformin, in patients with type 2 diabetes: a randomised, parallel-group, open-label trial. Int J Clin Pract. 2011;65:397–407.
Pratley RE, Nauck MA, Bailey T, et al. Efficacy and safety of switching from the DPP-4 inhibitor sitagliptin to the human GLP-1 analog liraglutide after 52 weeks in metformin-treated patients with type 2 diabetes: a randomized, open-label trial. Diabetes Care. 2012;35:1986–93.
Ahmann A, Rodbard HW, Rosenstock J, et al. Efficacy and safety of liraglutide versus placebo added to basal insulin analogues (with or without metformin) in patients with type 2 diabetes: a randomized, placebo-controlled trial. Diabetes Obes Metab. 2015;17:1056–64.
Fonseca VA, Devries JH, Henry RR, et al. Reductions in systolic blood pressure with liraglutide in patients with type 2 diabetes: insights from a patient-level pooled analysis of six randomized clinical trials. J Diabetes Complicat. 2014;28:399–405.
Lundershausen R, Müller S, Hashim M, Kienhöfer J, Kipper S, Wilke T. Quality of life, glycemic control, safety and tolerability associated with liraglutide or insulin initiation in patients with type 2 diabetes in Germany: results from the prospective, non-interventional LIBERTY study. Exp Clin Endocrinol Diabetes. 2020;128:170–81.
Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22.
Ostawal A, Mocevic E, Kragh N, Xu W. Clinical effectiveness of liraglutide in type 2 diabetes treatment in the real-world setting: a systematic literature review. Diabetes Ther. 2016;7:411–38.
Ali MS, Prieto-Alhambra D, Lopez LC, et al. Propensity score methods in Health Technology Assessment: principles, extended applications, and recent advances. Front Pharmacol. 2019;10:973.
Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32:84–90.
Gautier JF, Martinez L, Penfornis A, et al. Effectiveness and persistence with liraglutide among patients with type 2 diabetes in routine clinical practice–EVIDENCE: a prospective, 2-year follow-up, observational, post-marketing study. Adv Ther. 2015;32:838–53.
Buysschaert M, D'Hooge D, Preumont V, et al. ROOTS: a multicenter study in Belgium to evaluate the effectiveness and safety of liraglutide (Victoza®) in type 2 diabetic patients. Diabetes Metab Syndr. 2015;9:139–42.
Mezquita-Raya P, Reyes-Garcia R, Moreno-Perez O, et al. Clinical effects of liraglutide in a real-world setting in Spain: eDiabetes-Monitor SEEN Diabetes Mellitus Working Group Study. Diabetes Ther. 2015;6:173–85.
Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412–9.
Thayer S, Wei W, Buysman E, et al. The INITIATOR study: pilot data on real-world clinical and economic outcomes in US patients with type 2 diabetes initiating injectable therapy. Adv Ther. 2013;30:1128–40.
Buysman EK, Liu F, Hammer M, Langer J. Impact of medication adherence and persistence on clinical and economic outcomes in patients with type 2 diabetes treated with liraglutide: a retrospective cohort study. Adv Ther. 2015;32:341–55.
Wei W, Buysman E, Grabner M, et al. A real-world study of treatment patterns and outcomes in US managed-care patients with type 2 diabetes initiating injectable therapies. Diabetes Obes Metab. 2017;19:375–86.
Fabunmi R, Nielsen LL, Quimbo R, et al. Patient characteristics, drug adherence patterns, and hypoglycemia costs for patients with type 2 diabetes mellitus newly initiated on exenatide or insulin glargine. Curr Med Res Opin. 2009;25:777–86.
Misurski D, Lage MJ, Fabunmi R, Boye KS. A comparison of costs among patients with type 2 diabetes mellitus who initiated therapy with exenatide or insulin glargine. Appl Health Econ Health Policy. 2009;7:245–54.
Kiiskinen U, Matthaei S, Reaney M, et al. Resource use and costs of exenatide bid or insulin in clinical practice: the European CHOICE study. Clinicoecon Outcomes Res. 2013;5:355–567.
Baser O, Wei W, Baser E, Xie L. Clinical and economic outcomes in patients with type 2 diabetes initiating insulin glargine disposable pen versus exenatide BID. J Med Econ. 2011;14:673–80.
Mody R, Huang Q, Yu M, et al. Clinical and economic outcomes among injection-naïve patients with type 2 diabetes initiating dulaglutide compared with basal insulin in a US real-world setting: the DISPEL Study. BMJ Open Diabetes Res Care. 2019;7:e000884.
Wilke T, Picker N, Mueller S, et al. Real-world insulin therapy in German type 2 diabetes mellitus patients: patient characteristics, treatment patterns, and insulin dosage. Diabetes Metab Syndr Obes. 2019;12:1225–377.
Wilke T, Groth A, Fuchs A, et al. Real life treatment of diabetes mellitus type 2 patients: an analysis based on a large sample of 394,828 German patients. Diabetes Res Clin Pract. 2014;106:275–85.
Acknowledgements
Funding
This study and the journal’s Rapid Service Fee were funded by Novo Nordisk Pharma GmbH.
Medical Writing and Editorial Assistance
The authors thank Joachim Kienhöfer, Sebastian Pieperhoff, Elke Mathey (all from Novo Nordisk Pharma GmbH) and Michael Lyng Wolden (Nordisk A/S, Denmark) for their contribution towards the review of this manuscript. Medical writing assistance was provided by Lesley Taylor, on behalf of Watermeadow Medical, and Alice Singleton, of Watermeadow Medical, an Ashfield Company, part of UDG Healthcare plc, funded by Novo Nordisk Pharma GmbH (Mainz, Germany). Editing assistance was provided by Izabel James of Watermeadow Medical, funded by Novo Nordisk Pharma GmbH (Mainz, Germany).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Thomas Wilke has received consultant fees from the pharmaceutical companies Novo Nordisk, Merck, GSK, AstraZeneca, Takeda, Bayer and Boehringer Ingelheim. Sabrina Müller is an employee of Ingress-Health, which received funding from Novo Nordisk to support the conceptual design of the study and conduct the analysis. Andreas Fuchs has nothing to disclose. Margit S Kaltoft, Stefan Kipper, and Malgorzata Cel are employees of Novo Nordisk and have received shares from Novo Nordisk.
Compliance with Ethics Guidelines
Ethics approval was granted for the preceding LIBERTY study, which included approval for long-term (up to 5 years) data analysis of participating patients who had AOK PLUS health fund data available. Not all patients in the current study took part in the LIBERTY study. However, as the evaluation was based upon retrospective analysis of anonymised data from a health fund, it was not considered necessary to gain ethics approval or seek patient consent for the additional patients included in this analysis.
Data Availability
The aggregated reports can be shared upon reasonable request to the corresponding author; patient-level data cannot be shared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Digital Features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12765320.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wilke, T., Mueller, S., Fuchs, A. et al. Diabetes-Related Effectiveness and Cost of Liraglutide or Insulin in German Patients with Type 2 Diabetes: A 5-Year Retrospective Claims Analysis. Diabetes Ther 11, 2357–2370 (2020). https://doi.org/10.1007/s13300-020-00903-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-020-00903-0