Abstract
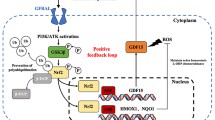
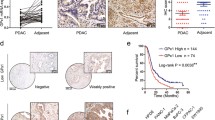
Therapeutic benefits offered by common chemotherapy drugs, such as oxaliplatin, are limited due to the development of resistance, which contributes to treatment failure and metastasis. The epithelial-mesenchymal transition (EMT) is a key event contributing to the development of resistance to chemotherapeutics. Although the relationship between oxaliplatin and chemotherapy resistance has been described for decades, the molecular mechanisms have remained elusive. The aim of the present study was to investigate the underlying mechanisms of oxaliplatin-mediated metastasis. Here, we identify reactive oxygen species (ROS) as mediators that promote the oxaliplatin-induced EMT. Following oxaliplatin treatment, the messenger RNA (mRNA) levels of most peroxiredoxin family genes, except for peroxiredoxin 1 (prdx1) gene, were constant or even decreased, resulting in ROS abundance. And the antioxidant guardian Nrf2 was unconspicuously raised both transcriptionally and translationally with oxaliplatin treatment as compared to those induced by topotecan treatment, which has been proved with no induced metastasis. In addition, the study evaluated high levels of ROS leading to EMT via activation of the known oncogenes Akt and Snail. Using the Akt inhibitor LY294002 or knocking down Snail expression via RNA interference (RNAi) reversed the effects of oxaliplatin on the EMT and metastasis. Our studies establish a role for the ROS-Akt-Snail axis as a mechanism by which chemotherapeutics induce EMT and cancer metastasis.






Similar content being viewed by others
Abbreviations
- CRC:
-
colorectal cancer
- EMT:
-
epithelial-mesenchymal transition
- NAC:
-
N-acetyl-L-cysteine
- ROS:
-
reactive oxygen species
References
Ciombor KK, Wu C, Goldberg RM. Recent therapeutic advances in the treatment of colorectal cancer. Annu Rev Med. 2015;66:83–95. doi:10.1146/annurev-med-051513-102539.
Sprowl JA, Ciarimboli G, Lancaster CS, Giovinazzo H, Gibson AA, Du G, et al. Oxaliplatin-induced neurotoxicity is dependent on the organic cation transporter OCT2. Proc Natl Acad Sci U S A. 2013;110(27):11199–204. doi:10.1073/pnas.1305321110.
Nativi C, Gualdani R, Dragoni E, Di Cesare ML, Sostegni S, Norcini M, et al. A TRPA1 antagonist reverts oxaliplatin-induced neuropathic pain. Sci Rep. 2013;3:2005. doi:10.1038/srep02005.
Watanabe T, Kobunai T, Yamamoto Y, Matsuda K, Ishihara S, Nozawa K, et al. Gene expression signature and response to the use of leucovorin, fluorouracil and oxaliplatin in colorectal cancer patients. Clin Transl Oncol. 2011;13(6):419–25. doi:10.1007/s12094-011-0676-z.
Lancaster CS, Sprowl JA, Walker AL, Hu S, Gibson AA, Sparreboom A. Modulation of OATP1B-type transporter function alters cellular uptake and disposition of platinum chemotherapeutics. Mol Cancer Ther. 2013;12(8):1537–44. doi:10.1158/1535-7163.MCT-12-0926.
Arriaga JM, Greco A, Mordoh J, Bianchini M. Metallothionein 1G and zinc sensitize human colorectal cancer cells to chemotherapy. Mol Cancer Ther. 2014;13(5):1369–81. doi:10.1158/1535-7163.MCT-13-0944.
Townsend DM, Tew KD. The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene. 2003;22(47):7369–75. doi:10.1038/sj.onc.1206940.
Kap EJ, Seibold P, Richter S, Scherer D, Habermann N, Balavarca Y et al. Genetic variants in DNA repair genes as potential predictive markers for oxaliplatin chemotherapy in colorectal cancer. The pharmacogenomics journal. 2015. doi:10.1038/tpj.2015.8.
Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68(10):3645–54. doi:10.1158/0008-5472.CAN-07-2938.
Barriere G, Fici P, Gallerani G, Fabbri F, Rigaud M. Epithelial mesenchymal transition: a double-edged sword. Clin Transl Med. 2015;4:14. doi:10.1186/s40169-015-0055-4.
Zhang P, Sun Y, Ma L. ZEB1: at the crossroads of epithelial-mesenchymal transition, metastasis and therapy resistance. Cell Cycle. 2015;14(4):481–7. doi:10.1080/15384101.2015.1006048.
Mikheeva SA, Mikheev AM, Petit A, Beyer R, Oxford RG, Khorasani L, et al. TWIST1 promotes invasion through mesenchymal change in human glioblastoma. Mol Cancer. 2010;9:194. doi:10.1186/1476-4598-9-194.
Kajiyama H, Shibata K, Terauchi M, Yamashita M, Ino K, Nawa A, et al. Chemoresistance to paclitaxel induces epithelial-mesenchymal transition and enhances metastatic potential for epithelial ovarian carcinoma cells. Int J Oncol. 2007;31(2):277–83.
Mitra A, Mishra L, Li S. EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget. 2015;6(13):10697–711.
Lim SO, Gu JM, Kim MS, Kim HS, Park YN, Park CK, et al. Epigenetic changes induced by reactive oxygen species in hepatocellular carcinoma: methylation of the E-cadherin promoter. Gastroenterology. 2008;135(6):2128–40. doi:10.1053/j.gastro.2008.07.027. 40 e1-8.
Gong C, Liu B, Yao Y, Qu S, Luo W, Tan W, et al. Potentiated DNA damage response in circulating breast tumor cells confers resistance to chemotherapy. J Biol Chem. 2015;290(24):14811–25. doi:10.1074/jbc.M115.652628.
Wu JB, Shao C, Li X, Li Q, Hu P, Shi C, et al. Monoamine oxidase A mediates prostate tumorigenesis and cancer metastasis. J Clin Invest. 2014;124(7):2891–908. doi:10.1172/JCI70982.
Gu Q, He Y, Ji J, Yao Y, Shen W, Luo J, et al. Hypoxia-inducible factor 1alpha (HIF-1alpha) and reactive oxygen species (ROS) mediates radiation-induced invasiveness through the SDF-1alpha/CXCR4 pathway in non-small cell lung carcinoma cells. Oncotarget. 2015;6(13):10893–907.
Kinugasa H, Whelan KA, Tanaka K, Natsuizaka M, Long A, Guo A et al. Mitochondrial SOD2 regulates epithelial-mesenchymal transition and cell populations defined by differential CD44 expression. Oncogene. 2015. doi:10.1038/onc.2014.449.
Cichon MA, Radisky DC. ROS-induced epithelial-mesenchymal transition in mammary epithelial cells is mediated by NF-kB-dependent activation of Snail. Oncotarget. 2014;5(9):2827–38.
Fong MY, Jin S, Rane M, Singh RK, Gupta R, Kakar SS. Withaferin A synergizes the therapeutic effect of doxorubicin through ROS-mediated autophagy in ovarian cancer. PLoS One. 2012;7(7):e42265. doi:10.1371/journal.pone.0042265.
Dewaele M, Maes H, Agostinis P. ROS-mediated mechanisms of autophagy stimulation and their relevance in cancer therapy. Autophagy. 2010;6(7):838–54.
Yang W, Zou L, Huang C, Lei Y. Redox regulation of cancer metastasis: molecular signaling and therapeutic opportunities. Drug Dev Res. 2014;75(5):331–41. doi:10.1002/ddr.21216.
Pirson M, Knoops B. Expression of peroxiredoxins and thioredoxins in the mouse spinal cord during embryonic development. J Comp Neurol. 2015;523(17):2599–617. doi:10.1002/cne.23807.
Kwak MK, Itoh K, Yamamoto M, Kensler TW. Enhanced expression of the transcription factor Nrf2 by cancer chemopreventive agents: role of antioxidant response element-like sequences in the nrf2 promoter. Mol Cell Biol. 2002;22(9):2883–92.
Li W, Kong AN. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol Carcinog. 2009;48(2):91–104. doi:10.1002/mc.20465.
Espinosa-Diez C, Miguel V, Mennerich D, Kietzmann T, Sanchez-Perez P, Cadenas S, et al. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015;6:183–97. doi:10.1016/j.redox.2015.07.008.
Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3(3):155–66. doi:10.1038/nrm757.
Li QQ, Xu JD, Wang WJ, Cao XX, Chen Q, Tang F, et al. Twist1-mediated adriamycin-induced epithelial-mesenchymal transition relates to multidrug resistance and invasive potential in breast cancer cells. Clin Cancer Res. 2009;15(8):2657–65. doi:10.1158/1078-0432.CCR-08-2372.
Leslie NR, Bennett D, Lindsay YE, Stewart H, Gray A, Downes CP. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 2003;22(20):5501–10. doi:10.1093/emboj/cdg513.
Grille SJ, Bellacosa A, Upson J, Klein-Szanto AJ, van Roy F, Lee-Kwon W, et al. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 2003;63(9):2172–8.
Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, et al. Colorectal cancer. Lancet. 2010;375(9719):1030–47. doi:10.1016/S0140-6736(10)60353-4.
Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7(8):573–84. doi:10.1038/nrc2167.
Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22(1):23–30. doi:10.1200/JCO.2004.09.046.
Giacchetti S, Perpoint B, Zidani R, Le Bail N, Faggiuolo R, Focan C, et al. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000;18(1):136–47.
Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27(5):663–71. doi:10.1200/JCO.2008.20.8397.
Poston GJ, Figueras J, Giuliante F, Nuzzo G, Sobrero AF, Gigot JF, et al. Urgent need for a new staging system in advanced colorectal cancer. J Clin Oncol. 2008;26(29):4828–33. doi:10.1200/JCO.2008.17.6453.
Martinez-Balibrea E, Martinez-Cardus A, Gines A, Ruiz de Porras V, Moutinho C, Layos L. Tumor-related molecular mechanisms of oxaliplatin resistance. Mol Cancer Ther. 2015;14(8):1767–76. doi:10.1158/1535-7163.MCT-14-0636.
Yang AD, Fan F, Camp ER, van Buren G, Liu W, Somcio R, et al. Chronic oxaliplatin resistance induces epithelial-to-mesenchymal transition in colorectal cancer cell lines. Clin Cancer Res. 2006;12(14 Pt 1):4147–53. doi:10.1158/1078-0432.CCR-06-0038.
Zheng HX, Cai YD, Wang YD, Cui XB, Xie TT, Li WJ, et al. Fas signaling promotes motility and metastasis through epithelial-mesenchymal transition in gastrointestinal cancer. Oncogene. 2013;32(9):1183–92. doi:10.1038/onc.2012.126.
Nagano O, Okazaki S, Saya H. Redox regulation in stem-like cancer cells by CD44 variant isoforms. Oncogene. 2013;32(44):5191–8. doi:10.1038/onc.2012.638.
Wu WS. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006;25(4):695–705. doi:10.1007/s10555-006-9037-8.
Lee DJ, Kang SW. Reactive oxygen species and tumor metastasis. Mol Cell. 2013;35(2):93–8. doi:10.1007/s10059-013-0034-9.
He T, Hatem E, Vernis L, Huang ME. Peroxiredoxin 1 knockdown sensitizes cancer cells to reactive oxygen species-generating drugs—an alternative approach for chemotherapy. Free Radic Biol Med. 2014;75 Suppl 1:S13. doi:10.1016/j.freeradbiomed.2014.10.583.
Acknowledgments
This work was supported by grants from the National Nature Science Foundation of China (81272341), the Fundamental Research Funds for the Central Universities (12ykpy50), and the Pearl River Nova Program of Guangzhou (2014J220039).
Authors’ contributions
LJ carried out the immunoassays, performed the statistical analysis, and drafted the manuscript. D-DL carried out the microscope observation and flow cytometry assay and participated in the design of the study. C-LY, R-QP, and Y-QG participated in the immunohistochemistry assay. X-SZ and X-FZ conceived of the study and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Compliance with ethical standards
All human experiments complied with ethical regulations and were approved by the medical ethics subcommittee of Sun Yat-sen University.
Conflicts of interest
None.
Additional information
Lin Jiao and Dan-Dan Li contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.

Suppl Fig. 1
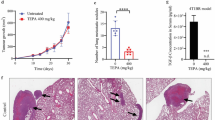
Oxaliplatin Induced EMT and Invasion (a) HCT116 cells were treated with five clinical drugs for indicated concentration respectively. E-cadherin and Vimentin expression were examined by western blotting. (b) the migrating numbers of cells treated with five clinical drugs were counted, respectively (mean ± SD in three separate experiments in triplicates). (GIF 131 kb)
Rights and permissions
About this article
Cite this article
Jiao, L., Li, DD., Yang, CL. et al. Reactive oxygen species mediate oxaliplatin-induced epithelial-mesenchymal transition and invasive potential in colon cancer. Tumor Biol. 37, 8413–8423 (2016). https://doi.org/10.1007/s13277-015-4736-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4736-9