Abstract
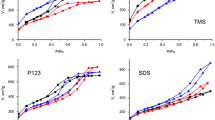
Organosilica materials with mesoporous structure were prepared by hydrothermal sol–gel condensation of tetraethyl orthosilicate and (3-aminopropyl)triethoxysilane in the presence of cetyltrimethylammonium and decyltrimethylammonium bromide as pore-generating agents. The effect of azo dyes, alizarin yellow, and methyl red as cosurfactants and their alkoxysilane derivatives as structure-forming silanes on mesostructure and morphology of resulting silicas was studied. The mesoporous structure of synthesized materials was characterized by low-temperature adsorption–desorption of nitrogen, X-ray diffraction analysis, and transmission electron microscopy. It was confirmed that the pore size, specific surface area, and hexagonal arrangement of mesopores in synthesized silica nanoparticles were strongly affected by the azo dye additives. Solubilization of azo dyes by liquid crystal phase of long-chain alkyltrimethylammonium salts and cooperative organization of dye-containing silanes with surfactants, involving penetration of aromatic groups of silanes into micelles, causes formation of more uniform mesoporous structure. As it was elucidated from the analysis of low-temperature adsorption–desorption of nitrogen, introduction of azo dye additives in sol–gel process results in noticeable increase of surface area, narrowing of pore size distribution and pore diameter. In accordance with the results of X-ray powder diffraction analysis, formation of silica materials which are characterized by the more distinct long-range ordering of porous structure takes place. The presence of azo dye compounds in sol–gel reaction mixture does not influence the morphology of resulting aminopropyl silica nanoparticles in noticeable extent, but causes formation of highly ordered arrays of cylindrical mesopores.








Similar content being viewed by others
References
Alireza B, Razieh V, Abed H (2008) Study of silanolate groups (≡ SiO–) in synthesis of micelle templated silica with various condition of cationic surfactant. Iran J Chem Chem Eng 27(1):1–6
Alothman ZA (2012) A review: fundamental aspects of silicate mesoporous materials. Materials 5:2874–2902
Barrett EP, Joyner LG, Halenda PH (1951) The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J Am Chem Soc 73(1):373–380
Beck JJS, Vartuli JC, Roth WJ, Leonowicz ME, Kresge CT, Schmitt KD, Chu CT-W, Olson DH, Sheppard EW, McCullen SB, Higgins JB, Schlenkert JL (1992) A new family of mesoporous molecular sieves prepared with liquid crystal templates. J Am Chem Soc 114:10834–10843
Belyakova LA, Besarab LN, Roik NV, Lyashenko DY, Vlasova NN, Golovkova LP, Chuiko AA (2006) Designing of the centers for adsorption of bile acids on a silica surface. J Colloid Interface Sci 294:11–20
Blin JL, Su BL (2002) Tailoring pore size of ordered mesoporous silicas using one or two organic auxiliaries as expanders. Langmuir 18:5303–5308
Bragg WL (1913) The diffraction of short electromagnetic waves by a crystal. Proc Camb Philos Soc 17:43–57
Cho YM, Lee WK, Kim B-K (1981) Studies on the interaction of azo dyes with cationic surfactant (I). Arch Pharm Res 4(2):75–84
Fenelonov VB, Romannikov VN, Derevyankin AY (1999) Mesopore size and surface area calculations for hexagonal mesophases (types MCM-41, FSM-16, etc.) using low-angle XRD and adsorption data. Micropor Mesopor Mater 28:57–72
Gehlen MH, Ferreira M, Neumann MG (1995) Interaction of methyl orange with cationic micelles and its effect on dye photochemistry. J Photochem Photobiol A 87:55–60
Gregg SJ, Sing KSW (1967) Adsorption, surface area and porosity. Academic Press, London
Hosseinzadeh R, Maleki R, Matin AA, Nikkhahi Y (2008) Spectrophotometric study of anionic azo-dye light yellow (X6G) interaction with surfactants and its micellar solubilization in cationic surfactant micelles. Spectrochim Acta A 69:1183–1187
Jana SK, Mochizuki A, Namba S (2004) Progress in pore-size control of mesoporous MCM41 molecular sieve using surfactant having different alkyl chain lengths and various organic auxiliary chemicals. Catal Surv Asia 8(1):1–13
Kresge CT, Leonowicz ME, Roth WJ, Vartuli JC, Beck JS (1992) Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 359:710–712
Lee H-H, Shibata J, Ahn J-W, Kim H (2008) Incorporation of short chain alkylamine in template micelle during MCM-41 formation process. Mater Trans 49(3):565–571
Lin H-P, Cheng Y-R, Liu S-B, Mou C-Y (1999) The effect of alkan-1-ols addition on the structural ordering and morphology of mesoporous silicate MCM-41. J Mater Chem 9:1197–1201
Liu S, Cool P, Collart O, Van Der Voort P, Vansant EF, Lebedev OI, Van Tendeloo G, Jiang M (2003) The influence of the alcohol concentration on the structural ordering of mesoporous silica: cosurfactant versus cosolvent. J Phys Chem B 107:10405–10411
Liu C, Wang X, Lee S, Pfefferle LD, Haller GL (2012) Surfactant chain length effect on the hexagonal-to-cubic phase transition in mesoporous silica synthesis. Micropor Mesopor Mater 147:242–251
Melendez-Ortiz HI, Mercado-Silva A, Garcia-Cerda LA, Castruita G, Perera-Mercado YA (2013) Hydrothermal synthesis of mesoporous silica MCM-41 using commercial sodium silicate. J Mex Chem Soc 57(2):73–79
Namba S, Mochizuki A (1998) Effect of auxiliary chemicals on preparation of silica MCM-41. Res Chem Intermed 24(5):561–570
Namba S, Mochizuki A, Kito M (1998) Preparation of highly ordered MCM-41 with docosyltrimethylammonium chloride (C22TMAC1) as a template and fine control of its pore size. Stud Surf Sci Catal 117:257–264
Nazar MF, Shah SS, Khosa MA (2010) Interaction of azo dye with cationic surfactant under different pH conditions. J Surfactants Deterg 13(4):529–537
Neimark AV, Ravikovitch PI, Grun M, Schuth F, Unger KK (1998) Pore size analysis of MCM-41 type adsorbents by means of nitrogen and argon adsorption. J Colloid Interface Sci 207:159–169
Ogawa M (2017) Mesoporous silica layer: preparation and opportunity. Chem Rec 17(2):217–232
Putz A-M, Cecilia S, Ianaşi C, Dudas Z, Szekely KN, Plocek J, Sfarloaga P, Sacarescu L, Almasy L (2015) Pore ordering in mesoporous matrices induced by different directing agents. J Porous Mater 22:321–331
Qu Q, Zhou G, Ding Y, Feng S, Gu Z (2014) Adjustment of the morphology of MCM-41 silica in basic solution. J Non Cryst Solids 405:104–115
Reinsborough VC, Holzwart JF (1986) Kinetics of the interactions between dyes and micelles. Can J Chem 64:955–959
Rottman C, Turniansky A, Avnir D (1998) Sol-gel physical and covalent entrapment of three methyl red indicators: a comparative study. J Sol Gel Sci Technol 13(1–3):17–25
Sajjadi SA, Izadbakhsh A, Niknam K (2016) Effect of synthesis conditions on textural properties of silica MCM-41. J Oil Gas Petrochem Technol 3(1):59–82
Sayari A, Yang Y, Kruk M, Jaroniec M (1999) Expanding the pore size of MCM-41 silicas: use of amines as expanders in direct synthesis and postsynthesis procedures. J Phys Chem B 103:3651–3658
Sun Y, Han S, Yu X, Che H, Liu A, Wang S (2010) Synthesis of highly ordered supermicroporous silica using short-chain cationic trimeric surfactant as structure-directing agent. J Porous Mater 17:597–603
Tanaka M, Itadani A, Kuroda Y, Iwamoto M (2012) Effect of pore size and nickel content of Ni–MCM-41 on catalytic activity for ethene dimerization and local structures of nickel ions. J Phys Chem C 116:5664–5672
Ulagappan N, Rao CNR (1996) Evidence for supramolecular organization of alkane and surfactant molecules in the process of forming mesoporous silica. Chem Commun 24:2759–2760
Xu R, Pang W, Yu J, Huo Q, Chen J (2007) Chemistry of zeolites and related porous materials: synthesis and structure. Wiley, Singapore
Yanagisawa T, Shimizu T, Kuroda K, Kato C (1990) The preparation of alkyltriinethylaininonium–kaneinite complexes and their conversion to microporous materials. Bull Chem Soc Jpn 63:988–992
Zhang H, Li X (2016) Novel mesoporous silica materials with hierarchically ordered nanochannel: synthesis with the assistance of straight-chain alkanes and application. J Chem 2016:1–16
Zhao XS, Lu GQ, Millar GJ (1996) Advances in mesoporous molecular sieve MCM-41. Ind Eng Chem Res 35:2075–2090
Funding
We received no funding for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Roik, N.V., Belyakova, L.A., Dziazko, M.O. et al. Influence of azo dye additives on structural ordering of mesoporous silicas. Appl Nanosci 10, 2547–2556 (2020). https://doi.org/10.1007/s13204-019-01013-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-019-01013-5