Abstract
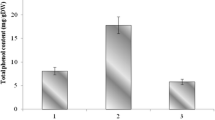
Rumex dentatus L. (Family: Polygonaceae) is a weedy plant widely distributed in many countries including Egypt. It has been used in the Mediterranean diet as a leafy vegetable and its leaves and roots exhibited various biological activities. In our study, total phenolics, antioxidant capacities assayed by 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging and β-carotene bleaching methods and reducing power were evaluated in different extracts/fractions of leaves and roots of R. dentatus grown in Egypt. In addition, their phenolic compositions were determined by GC-MS and HPLC. The results showed that total phenolic content in the ethyl acetate fractions of leaves and roots were high and measured at 169.5 and 257.4 mg gallic acid equivalent per g extract, respectively. The ethyl acetate fractions of leaves and roots exhibited strong DPPH activity and the DPPH IC50 values were 0.021 and 0.012 mg mL−1 of leaves and roots, respectively. Furthermore, the ethyl acetate fractions of leaves and roots showed high reducing power and antioxidant activity assayed by β-carotene bleaching method. GC-MS and HPLC analyses indicated that these fractions contained a variety of phenolic compounds including p-hydroxybenzoic acid, syringic acid, vanillin, benzoic acid, ferulic acid, and cinnamic acid. Our study verified that the ethyl acetate fractions of leaves and roots of R. dentatus have strong antioxidant activities which are correlated with its high levels of phenolic compounds and therefore, they could be utilized as a natural source of antioxidant in food industry.
Similar content being viewed by others
References
Abe N, Murata T, Hirota A. 1998. Novel DPPH radical scavengers, bisorbicillinol and demethyltrichodimerol, from a fungus. Biosci. Biotechnol. Biochem. 62: 661–666
Ali M, Arfan M, Ahmad H, Zaman K, Khan F, Amarowicz R. 2011. Comparative antioxidant and antimicrobial activities of phenolic compounds extracted from five Hypericum species. Food Technol. Biotechnol. 49: 205–213
Barros L, Cabrita L, Boas MV, Carvalho AM, Ferreira ICFR. 2011. Chemical, biochemical and electrochemical assays to evaluate phytochemicals and antioxidant activity of wild plants. Food Chem. 127: 1600–1608
Blois MS. 1958. Antioxidant determinations by the use of a stble free radical. Nature 181: 1199–1200
Çaliskan O, Polat AA. 2011. Phytochemical and antioxidant properties of selected fig (Ficus carica L.) accessions from the eastern Mediterranean region of Turkey. Sci. Hortic. 128: 473–478
Chua M, Tung Y, Chang S. 2008. Antioxidant activities of ethanolic extracts from the twigs of Cinnamomum osmophloeum. Bioresour. Technol. 99: 1918–1925
Elzaawely AA, Xuan TD, Tawata S. 2005. Antioxidant and anti bacterial activities of Rumex japonicus HOUTT. aerial parts. Biol. Pharm. Bull. 28: 2225–2230
Fatima N, Zia M, Riaz-ur-Rehman, Rizvil ZF, Ahmad S, Mirza B, Chaudhary MF. 2009. Biological activities of Rumex dentatus L: Evaluation of methanol and hexane extracts. Afr. J. Biotechnol. 8: 6945–6951
Germanò MP, D’Angelo V, Biasini T, Sanogo R, De Pasquale R, Catania S. 2006. Evaluation of the antioxidant properties and bioavailability of free and bound phenolic acids from Trichilia emetic Vahl. J. Ethnopharmacol. 105: 368–373
Hadjichambis ACH, Paraskeva-Hadjichambi D, Della A, Giusti ME, De Pasquale C et al. 2008. Wild and semi-domesticated food plant consumption in seven circum-Mediterranean areas. Int. J. Food Sci. Nutr. 59: 383–414
Hameed I, Dastagir G. 2009. Nutritional analyses of Rumex hastatus D. Don, Rumex dentatus Linn and Rumex nepalensis Spreng. Afr. J. Biotechnol. 8: 4131–4133
Hussain F, Ahmad B, Hameed I, Dastagir G, Sanaullah P, Azam S. 2010. Antibacterial, antifungal and insecticidal activities of some selected medicinal plants of Polygonaceae. Afr. J. Biotechnol. 9: 5032–5036
Hussain F, Mobeen F, Kil B, Yoo SO. 1997. Allelopathic suppression of wheat and mustard by Rumex dentatus ssp. klotzschianus. J. Plant Biol. 40: 120–124
Jayaprakasha GK, Singh RP, Sakariah, KK. 2001. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem. 73: 285–290
Kähkönen MP, Hopia AI, Vuorela HJ, Rauha J, Pihlaja K, Kujala TS. 1999. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 47: 3954–3962
Kumaran A, Karunakaran RJ. 2006. Antioxidant and free radical scavenging activity of an aqueous extract of Coleus aromaticus. Food Chem. 97: 109–114
Li C, Liu S. 2009. Screening of Chinese plant extracts for antioxidant activity. Mod. Pharm. Res. 2: 31–35
Liu SY, Sporer F, Wink M, Jourdane J, Henning R, Li YL, Ruppel A. 1997. Anthraquinones in Rheum palmatum and Rumex dentatus (Polygonaceae), and phorbol esters in Jatropha curcas (Euphorbiaceae) with molluscicidal activity against the schistosome vector snails Oncomelania, Biomphalaria and Bulinus. Trop. Med. Int. Health. 2: 179–188
Loganayaki N, Siddhuraju P, Manian S. 2011. Antioxidant activity and free radical scavenging capacity of phenolic extracts from Helicteres isora L. and Ceiba pentandra L. J. Food Sci. Technol. DOI 10.1007/s13197-011-0389-x
Madhujith T, Shahidi F. 2009. Antioxidant potential of barley as affected by alkaline hydrolysis and release of insolublebound phenolics. Food Chem. 117: 615–620
Mariod AA, Ibrahim RM, Ismail M, Ismail N. 2009. Antioxidant activity and phenolic content of phenolic rich fractions obtained from black cumin (Nigella sativa) seedcake. Food Chem. 116: 306–312
Mariod AA, Ibrahim RM, Ismail M, Ismail N. 2010. Antioxidant activities of phenolic rich fractions (PRFs) obtained from black mahlab (Monechma ciliatum) and white mahlab (Prunus mahaleb) seedcakes. Food Chem. 118: 120–127
Mensor LL, Menezes FS, Leitão GG, Reis AS, dos Santos TC, Coube CS, Leitão SG. 2001. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 15: 127–130
Pourmorad F, Hosseinimehr SJ, Shahabimajd N. 2006. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr. J. Biotechnol. 5: 1142–1145
Shih FF, Diagle KW. 2003. Antioxidant properties of milledrice co-products and their effects on lipid oxidation in ground beef. Food Chem. Toxicol. 68: 2672–2675
Siddhuraju P, Becker K. 2003. Studies on antioxidant activities of mucuna seed (Mucuna pruriens var utilis) extract and various nonprotein amino/imino acids through in vitro models. J. Sci. Food Agric. 83: 1517–1524
Soares AA, de Souza CGM, Daniel FM, Ferrari GP, da Costa SMG, Peralta RM. 2009. Antioxidant activity and total phenolic content of Agaricus brasiliensis (Agaricus blazei Murril) in two stages of maturity. Food Chem. 112: 775–781
Su X, Wang Z, Liu J. 2009. In vitro and in vivo antioxidant activity of Pinus koraiensis seed extract containing phenolic compounds. Food Chem. 117: 681–686
Umer A, Yousaf Z, Khan F, Hussain U, Anjum A, Nayyab Q, Younas A. 2010. Evaluation of allelopathic potential of some selected medicinal species. Afr. J. Biotechnol. 9: 6194–6206
Yildirim A, Mavi A, Kara AA. 2003. Antioxidant and antimicrobial activities of Polygonum cognatum Meissn extracts. J. Sci. Food Agric. 83: 64–69
Zhang Q, Zhang Z, Cheung H. 2009. Antioxidant activity of Rhizoma Smilacis Glabrae extracts and its key constituentastilbin. Food Chem. 115: 297–303
Zhang Z, Liao L, Moore J, Wu T, Wang Z. 2009. Antioxidant phenolic compounds from walnut kernels (Juglans regia L.). Food Chem. 113: 160–165
Zhu JJ, Zhang CF, Zhang M, Wang ZT. 2006a. Anthraquinones and chromones from Rumex dentatus. Biochem. Syst. Ecol. 34: 753–756
Zhu JJ, Zhang CF, Zhang M, Wang ZT. 2006b. Studies on chemical constituents in roots of Rumex dentatus. Zhongguo Zhongyao Zazhi. 31: 1691–1693
Author information
Authors and Affiliations
Corresponding author
Additional information
The work was done in the Department of Bioscience and Biotechnology, Faculty of Agriculture, University of the Ryukyus, Okinawa, Japan.
Rights and permissions
About this article
Cite this article
Elzaawely, A.A., Tawata, S. Antioxidant capacity and phenolic content of Rumex dentatus L. Grown in Egypt. J. Crop Sci. Biotechnol. 15, 59–64 (2012). https://doi.org/10.1007/s12892-011-0063-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12892-011-0063-x