Abstract
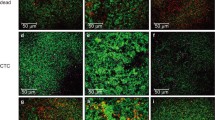
In this study, we report on the formation and resilience of Lactobacillus reuteri HFI-LD5 and Lactobacillus rhamnosus HFI-K2 biofilms cultivated in a CO2 evolution measurement system (CEMS) and exposed to biologically relevant, fasting-state gastrointestinal fluids under continuous flow conditions. For comparative purposes, planktonic and sessile populations of L. reuteri HFI-LD5 and L. rhamnosus HFI-K2 were each exposed to fasting-state gastric fluid (FSGF, pH 2.0) for 2 h, fasting-state intestinal fluid (FSIF, pH 7.5) for 6 h, and simulated colonic fluid (SCoF, pH 7.0) for 24 h. Planktonic cell numbers of L. reuteri HFI-LD5 declined from 6.6 log10 CFU/mL to 3.2 log10 CFU/mL and L. rhamnosus HFI-K2 from 6.6 log10 CFU/mL to undetectable levels after exposure to FSGF. Limited loss in viability was observed when free-floating cells were exposed to FSIF and SCoF. Sessile populations of both strains survived and recovered from the sequential exposure to all three gastric fluids despite observed detachment of biofilm biomass and a temporary decrease in metabolic activity to below detection limits, as recorded by changes in whole-biofilm CO2 production rates. The planktonic cell-focused gut microbiome-related research has most likely caused an underestimation in the overall survival ability of microorganisms in the gastrointestinal tract. Sessile cells of L. reuteri HFI-LD5 were metabolically inactive when exposed to gastric (FSGF) and intestinal (FSIF) fluids, suggesting that biofilms are formed in the small intestinal tract as survival mechanism. In the case of L. rhamnosus HFI-K2, cells were released from biofilms when suddenly exposed to pH 2.0.




Similar content being viewed by others
References
Wallace TC (2015) Dietary supplements in health promotion. CRC Press, Taylor & Francis
Gratz SW, Mykkanen H, El-Nezami HS (2010) Probiotics and gut health: a special focus on liver diseases. World J Gastroenterol 16:403–410
Hemarajata P, Versalovic J (2012) Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol 6:39–51
Kelesidis T, Pothoulakis C (2012) Efficacy and safety of the probiotic Saccharomyces boulardii for the prevention and therapy of gastrointestinal disorders. Therap Adv Gastroenterol 5:111–125
Schultz M (2008) Clinical use of Escherichia coli Nissle 1917 in inflammatory bowel disease. Inflamm Bowel Dis 14:1012–1018
Darilmaz DO, Beyatli Y (2012) Acid-bile, antibiotic resistance and inhibitory properties of Propionibacteria isolated from Turkish traditional home-made cheeses. Anaerobe 18:122–127
Cotter PD, Hill C (2003) Surviving the acid test: responses of Gram-positive bacteria to low pH. Microbiol Mol Biol Rev 67:429–453
Ruiz L, Margolles A, Sánchez B (2013) Bile resistance mechanisms in Lactobacillus and Bifidobacterium. Front Microbiol 4:396
FAO/WHO working group (2001) Report of the joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria, Córdoba, Argentina, 1–4 October (Food and Agriculture Organization of the United Nations)
Wichmann A, Allahyar A, Greiner TU, Plovier H, Lundén GÖ, Larsson T, Drucker DJ, Delzenne NM, Cani PD, Bäckhed F (2013) Microbial modulation of energy availability in the colon regulates intestinal transit. Cell Host Microbe 14:582–590
Belzer C, de Vos WM (2012) Microbes inside-from diversity to function: the case of Akkermansia. ISME J 6:1449–1458
Guerra A, Etienne-Mesmin L, Livrelli V, Denis S, Blanquet-Diot S, Alric M (2012) Relevance and challenges in modeling human gastric and small intestinal digestion. Trends Biotechnol 30:591–600
Minekus M, Alminger M, Alvito P, Ballance S, Bohn T, Bourlieu C, Carrière F, Boutrou R, Corredig M, Dupont D, Dufour C, Egger L, Golding M, Karakaya S, Kirkhus B, Le Feunteun S, Lesmes U, Macierzanka A, Mackie A, Marze S, McClements DJ, Ménard O, Recio I, Santos CN, Singh RP, Vegarud GE, Wickham MS, Weitschies W, Brodkorb A (2014) A standardised static in vitro digestion method suitable for food—an international consensus. Food Funct 5:1113–1124
Read NW, Al-Janabi MN, Holgate AM, Barber DC, Edwards CA (1986) Simultaneous measurement of gastric emptying, small bowel residence and colonic filling of a solid meal by the use of the gamma camera. Gut 27:300–308
Mudie DM, Amidon GL, Amidon GE (2010) Physiological parameters for oral delivery and in vitro testing. Mol Pharm 7:1388–1405
Kim ER, Rhee P-L (2012) How to interpret a functional or motility test—colon transit study. J Neurogastroenterol Motil 18:94–99
Wagener S, Shankar K, Turnock R, Lamont G, Baillie C (2004) Colonic transit time—what is normal? J Pediatr Surg 39:166–169
Nugent SG, Kumar D, Rampton DS, Evans DF (2001) Intestinal luminal pH in inflammatory bowel disease: possible determinants and implications for therapy with aminosalicylates and other drugs. Gut 48:571–577
Kennedy P, Brammah S, Wills E (2010) Burns, biofilm and a new appraisal of burn wound sepsis. Burns 36:49–56
Stewart PS, Costerton JW (2001) Antibiotic resistance of bacteria in biofilms. Lancet 358:135–138
Bollinger R, Barbas AS, Bush EL, Lin SS, Parker W (2007) Biofilms in the large bowel suggest an apparent function of the human vermiform appendix. J Theor Biol 249:826–831
Macfarlane S, Dillon JF (2007) Microbial biofilms in the human gastrointestinal tract. J Appl Microbiol 102:1187–1196
de Vos WM (2015) Microbial biofilms and the human intestinal microbiome. npj Biofilms Microbiomes 1:15005
Macfarlane S, Bahrami B, Macfarlane GT (2011) Mucosal biofilm communities in the human intestinal tract. Adv Appl Microbiol 75:111–143
Probert HM, Gibson GR (2002) Bacterial biofilms in the human gastrointestinal tract. Issues Intest Microbiol 3:23–27
Valeur N, Engel P, Carbajal N, Connolly E, Ladefoged K (2004) Colonization and immunomodulation by Lactobacillus reuteri ATCC 55730 in the human gastrointestinal tract. Appl Environ Microbiol 70:1176–1181
Lebeer S, Verhoeven TL, Claes IJ, De Hertogh G, Vermeire S, Buyse J, Van Immerseel F, Vanderleyden J, De Keersmaecker SC (2011) FISH analysis of Lactobacillus biofilms in the gastrointestinal tract of different hosts. Lett Appl Microbiol 52:220–226
Segers ME, Lebeer S (2014) Towards a better understanding of Lactobacillus rhamnosus GG-host interactions. Microb Cell Factories 13:S7
Lebeer S, Verhoeven TLA, Perea Vélez M, Vanderleyden J, De Keersmaecker SCJ (2007) Impact of environmental and genetic factors on biofilm formation by the probiotic strain Lactobacillus rhamnosus GG. Appl Environ Microbiol 73:6768–6775
Hou C, Zeng X, Yang F, Liu H, Qiao S (2015) Study and use of the probiotic Lactobacillus reuteri in pigs: a review. J Anim Sci Biotechnol 6:14
Walter J (2008) Ecological role of lactobacilli in the gastrointestinal tract: implications for fundamental and biomedical research. Appl Environ Microbiol 74:4985–4996
Ho M, Chang YY, Chang WC, Lin HC, Wang MH, Lin WC, Chiu TH (2016) Oral Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 to reduce group B Streptococcus colonization in pregnant women: a randomized controlled trial. Taiwan J Obstet Gynecol 55:515–518
Martinez RCR, Seney SL, Summers KL, Nomizo A, De Martinis ECP, Reid G (2009) Effect of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 on the ability of Candida albicans to infect cells and induce inflammation. Microbiol Immunol 53:487–495
Hekmat S, Soltani H, Reid G (2009) Growth and survival of Lactobacillus reuteri RC-14 and Lactobacillus rhamnosus GR-1 in yogurt for use as a functional food. Innov Food Sci Emerg Technol 10:293–296
Hsieh M, Munch E, Reid G, Roth D, Trautner B, Kaplan S, Jones E, Versalovic J (2009) Probiotic Lactobacillus reuteri RC-14 and Lactobacillus rhamnosus GR-1 for prevention of urinary tract infections in catheterization-dependent girls with spina bifida. J Pediatr Urol 5:47
Kroukamp O, Wolfaardt GM (2009) CO2 production as an indicator of biofilm metabolism. Appl Environ Microbiol 75:4391–4397
Klopper KB, Deane SM, Dicks LMT (2018) Aciduric strains of Lactobacillus reuteri and Lactobacillus rhamnosus, isolated from human feces, have strong adhesion and aggregation properties. Probiotics and Antimicro Prot 10:89–97
De Man JD, Rogosa M, Sharpe ME (1960) A medium for the cultivation of lactobacilli. J Appl Bacteriol 23:130–135
Marques MRC, Loebenberg R, Almukainzi M (2011) Simulated biological fluids with possible application in dissolution testing. Dissolution Technol 18:15–28
Salminen S (2012) Lactic acid bacteria: microbiological and functional aspects. (CRC Press, Taylor & Francis)
Rattanaprasert M, Roos S, Hutkins RW, Walter J (2014) Quantitative evaluation of synbiotic strategies to improve persistence and metabolic activity of Lactobacillus reuteri DSM 17938 in the human gastrointestinal tract. J Funct Foods 10:85–94
Bester E, Edwards EA, Wolfaardt GM (2009) Planktonic cell yield is linked to biofilm development. Can J Microbiol 55:1195–1206
Reuter G (2001) The Lactobacillus and Bifidobacterium microflora of the human intestine: composition and succession. Curr Issues Intest Microbiol 2:43–53
Frese S, Hutkins R, Walter J (2012) Comparison of the colonization ability of autochthonous and allochthonous strains of lactobacilli in the human gastrointestinal tract. Adv Microbiol 2:399–409
Walter J, Britton RA, Roos S (2011) Host-microbial symbiosis in the vertebrate gastrointestinal tract and the Lactobacillus reuteri paradigm. Proc Natl Acad Sci U S A 108:4645–4652
Aoudia N, Rieu A, Briandet R, Deschamps J, Chluba J, Jego G, Garrido C, Guzzo J (2016) Biofilms of Lactobacillus plantarum and Lactobacillus fermentum: effect on stress responses, antagonistic effects on pathogen growth and immunomodulatory properties. Food Microbiol 53:51–59
Wall T, Båth K, Britton RA, Jonsson H, Versalovic J, Roos S (2007) The early response to acid shock in Lactobacillus reuteri involves the ClpL chaperone and a putative cell wall-altering esterase. Appl Environ Microbiol 73:3924–3935
Jones SE, Versalovic J (2009) Probiotic Lactobacillus reuteri biofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiol 9:1471–2180
Salas-Jara MJ, Ilabaca A, Vega M, García A (2016) Biofilm forming Lactobacillus: new challenges for the development of probiotics. Microorganisms 4(3):1–14
Ambalam P, Kondepudi KK, Nilsson I, Wadström T, Ljungh Å (2012) Bile stimulates cell surface hydrophobicity, Congo red binding and biofilm formation of Lactobacillus strains. FEMS Microbiol Lett 333:10e19
Frese SA, Benson AK, Tannock GW, Loach DM, Kim J, Zhang M, Oh PL, Heng NCK, Patil PB, Juge N, MacKenzie DA, Pearson BM, Lapidus A, Dalin E, Tice H, Goltsman E, Land M, Hauser L, Ivanova N, Kyrpides NC, Walter J (2011) The evolution of host specialization in the vertebrate gut symbiont Lactobacillus reuteri. PLoS Genet 7:e1001314
Walter J, Chagnaud P, Tannock GW, Loach DM, Bello FD, Jenkinson HF, Hammes WP, Hertel C (2005) A high-molecular-mass surface protein (Lsp) and methionine sulfoxide reductase B (MsrB) contribute to the ecological performance of Lactobacillus reuteri in the murine gut. Appl Environ Microbiol 71:979–986
Walter J, Schwab C, Loach DM, Gänzle MG, Tannock GW (2008) Glucosyltransferase A (GtfA) and inulosucrase (Inu) of Lactobacillus reuteri TMW1.106 contribute to cell aggregation, in vitro biofilm formation, and colonization of the mouse gastrointestinal tract. Microbiology 154:72–80
Roos S, Jonsson H (2002) A high-molecular-mass cell-surface protein from Lactobacillus reuteri 1063 adheres to mucus components. Microbiology 148:433–442
MacKenzie DA, Jeffers F, Parker ML, Vibert-Vallet A, Bongaerts RJ, Roos S, Walter J, Juge N (2010) Strain-specific diversity of mucus-binding proteins in the adhesion and aggregation properties of Lactobacillus reuteri. Microbiology 156:3368–3378
Etzold S, MacKenzie DA, Jeffers F, Walshaw J, Roos S, Hemmings AM, Juge N (2014) Structural and molecular insights into novel surface-exposed mucus adhesins from Lactobacillus reuteri human strains. Mol Microbiol 92:543–556
Sims IM, Frese SA, Walter J, Loach D, Wilson M, Appleyard K, Eason J, Livingston M, Baird M, Cook G, Tannock GW (2011) Structure and functions of exopolysaccharide produced by gut commensal Lactobacillus reuteri 100-23. ISME J 5:1115–1124
Frese SA, MacKenzie DA, Peterson DA, Schmaltz R, Fangman T, Zhou Y, Zhang C, Benson AK, Cody LA, Mulholland F, Juge N, Walter J (2013) Molecular characterization of host-specific biofilm formation in a vertebrate gut symbiont. PLoS Genet 9:e1004057
Pitino I, Randazzo CL, Cross KL, Parker ML, Bisignano C, Wickham MS, Mandalari G, Caggia C (2012) Survival of Lactobacillus rhamnosus strains inoculated in cheese matrix during simulated human digestion. Food Microbiol 31:57–63
Lebeer S, Claes IJJ, Verhoeven TLA, Shen C, Lambrichts I, Ceuppens JL, Vanderleyden J, De Keersmaecker SCJ (2008) Impact of luxS and suppressor mutations on the gastrointestinal transit of Lactobacillus rhamnosus GG. Appl Environ Microbiol 74:4711–4718
Morelli L (2000) In vitro selection of probiotic lactobacilli: a critical appraisal. Curr Issues Intest Microbiol 1:59–67
Olson ME, Ceri H, Morck DW, Buret AG, Read RR (2002) Biofilm bacteria: formation and comparative susceptibility to antibiotics. Can J Vet Res 66:86–92
Funding
This research is funded by The National Research Foundation (NRF) of South Africa.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Klopper, K.B., Bester, E., Deane, S.M. et al. Survival of Planktonic and Sessile Cells of Lactobacillus rhamnosus and Lactobacillus reuteri upon Exposure to Simulated Fasting-State Gastrointestinal Conditions. Probiotics & Antimicro. Prot. 11, 594–603 (2019). https://doi.org/10.1007/s12602-018-9426-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-018-9426-7