Abstract
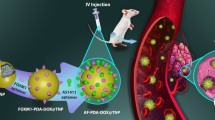
Osteosarcoma is the most common primary malignant neoplasm of the bone in children and adolescents and has a high risk of relapse and metastasis. Of the various methods to treat osteosarcoma, the use of genetic approaches to inhibit the rapid growth of osteosarcoma while limiting tumor metastasis has presented a challenge in its implementation. Here, we successfully synthesized a polysaccharide derivative (Amy-g-PLLD) for delivery of astrocyte elevated gene-1 (AEG-1) small-interfering RNA (siRNA) (siAEG-1), and used it for the first time to suppress osteosarcoma tumors in vitro and in vivo. Amy-g-PLLD/ siAEG-1 complexes were delivered into 143B human osteosarcoma cells with low resultant cytotoxicity. Osteosarcoma tumor proliferation and invasion were inhibited in vitro. Intratumoral injection of Amy-g-PLLD/siAEG-1 complexes markedly inhibited tumor growth and lung metastasis in 143B tumor-bearing mice. This biocompatible and effective approach employing a natural material-siRNA complex should pave the way for more genetic research in treating osteosarcoma.

Similar content being viewed by others
References
Mirabello, L.; Troisi, R. J.; Savage, S. A. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the surveillance, epidemiology, and end results program. Cancer 2009, 115, 1531–1543.
Stiller, C. A.; Bielack, S. S.; Jundt, G.; Steliarova-Foucher, E. Bone tumours in European children and adolescents, 1978–1997. Report from the automated childhood cancer information system project. Eur. J. Cancer 2006, 42, 2124–2135.
Bacci, G.; Briccoli, A.; Ferrari, S.; Saeter, G.; Donati, D.; Longhi, A.; Manfrini, M.; Bertoni, F.; Rimondini, S.; Monti, C. et al. Neoadjuvant chemotherapy for osteosarcoma of the extremities with synchronous lung metastases: Treatment with cisplatin, adriamycin and high dose of methotrexate and ifosfamide. Oncol. Rep. 2000, 7, 339–385.
Su, Z. Z.; Kang, D. C.; Chen, Y. M.; Pekarskaya, O.; Chao, W.; Volsky, D. J.; Fisher, P. B. Identification and cloning of human astrocyte genes displaying elevated expression after infection with HIV-1 or exposure to HIV-1 envelope glycoprotein by rapid subtraction hybridization, RaSH. Oncogene 2002, 21, 3592–3602.
Yoo, B. K.; Emdad, L.; Su, Z. Z.; Villanueva, A.; Chiang, D. Y.; Mukhopadhyay, N. D.; Mills, A. S.; Waxman, S.; Fisher, R. A.; Llovet, J. M. et al. Astrocyte elevated gene-1 regulates hepatocellular carcinoma development and progression. J. Clin. Invest. 2009, 119, 465–477.
Wang, F.; Ke, Z. F.; Sun, S. J.; Chen, W. F.; Yang, S. C.; Li, S. H.; Mao, X. P.; Wang, L. T. Oncogenic roles of astrocyte elevated gene-1 (AEG-1) in osteosarcoma progression and prognosis. Cancer Biol. Ther. 2011, 12, 539–548.
Sledz, C. A.; Williams, B. R. G. RNA interference in biology and disease. Blood 2005, 106, 787–794.
Anderson, W. F. Human gene therapy. Nature 1998, 392, 25–30.
Li, W. J.; Szoka, F. C. Jr. Lipid-based nanoparticles for nucleic acid delivery. Pharm. Res. 2007, 24, 438–449.
Daka, A.; Peer, D. RNAi-based nanomedicines for targeted personalized therapy. Adv. Drug Deliv. Rev. 2012, 64, 1508–1521.
Peer, D. Harnessing RNAi nanomedicine for precision therapy. Mol. Cell Ther. 2014, 2, 5.
Tian, H. Y.; Chen, J.; Chen, X. S. Nanoparticles for gene delivery. Small 2013, 9, 2034–2044.
Williford, J. M.; Wu, J.; Ren, Y.; Archang, M. M.; Leong, K. W.; Mao, H. Q. Recent advances in nanoparticlemediated siRNA delivery. Annu. Rev. Biomed. Eng. 2014, 16, 347–370.
Semple, S. C.; Akinc, A.; Chen, J. X.; Sandhu, A. P.; Mui, B. L.; Cho, C. K.; Sah, D. W.; Stebbing, D.; Crosley, E. J.; Yaworski, E. et al. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 2010, 28, 172–176.
Sato, Y.; Hatakeyama, H.; Sakurai, Y.; Hyodo, M.; Akita, H.; Harashima, H. A pH-sensitive cationic lipid facilitates the delivery of liposomal siRNA and gene silencing activity in vitro and in vivo. J. Control. Release 2012, 163, 267–276.
Elbakry, A.; Zaky, A.; Liebl, R.; Rachel, R.; Goepferich, A.; Breunig, M. Layer-by-layer assembled gold nanoparticles for siRNA delivery. Nano Lett. 2009, 9, 2059–2064.
Lei, Y. F.; Tang, L. X.; Xie, Y. Z. Y.; Xianyu, Y. L.; Zhang, L. M.; Wang, P.; Hamada, Y.; Jiang, K.; Zheng, W. F.; Jiang, X. Y. Gold nanoclusters-assisted delivery of NGF siRNA for effective treatment of pancreatic cancer. Nat. Commun. 2017, 8, 15130.
Xiao, Y. L.; Jaskula-Sztul, R.; Javadi, A.; Xu, W. J.; Eide, J.; Dammalapati, A.; Kunnimalaiyaan, M.; Chen, H.; Gong, S. Q. Co-delivery of doxorubicin and siRNA using octreotideconjugated gold nanorods for targeted neuroendocrine cancer therapy. Nanoscale 2012, 4, 7185–7193.
Sun, C. Y.; Shen, S.; Xu, C. F.; Li, H. J.; Liu, Y.; Cao, Z. T.; Yang, X. Z.; Xia, J. X.; Wang, J. Tumor acidity-sensitive polymeric vector for active targeted siRNA delivery. J. Am. Chem. Soc. 2015, 137, 15217–15224.
Cai, X. J.; Zhu, H. F.; Zhang, Y. M.; Gu, Z. W. Highly efficient and safe delivery of VEGF siRNA by bioreducible fluorinated peptide dendrimers for cancer therapy. ACS Appl. Mater. Interfaces 2017, 9, 9402–9415.
Zhang, N.; Chen, H.; Liu, A. Y.; Shen, J. J.; Shah, V.; Zhang, C.; Hong, J.; Ding, Y. Gold conjugate-based liposomes with hybrid cluster bomb structure for liver cancer therapy. Biomaterials 2016, 74, 280–291.
Zhao, X.; Li, F.; Li, Y. Y.; Wang, H.; Ren, H.; Chen, J.; Nie, G. J.; Hao, J. H. Co-delivery of HIF1α siRNA and gemcitabine via biocompatible lipid-polymer hybrid nanoparticles for effective treatment of pancreatic cancer. Biomaterials 2015, 46, 13–25.
Lee, J. M.; Yoon, T. J.; Cho, Y. S. Recent developments in nanoparticle-based siRNA delivery for cancer therapy. Biomed. Res. Int. 2013, 2013, 782041.
Singha, K.; Namgung, R.; Kim, W. J. Polymers in smallinterfering RNA delivery. Nucl. Acid Ther. 2011, 21, 133–147.
Merdan, T.; Kopecek, J.; Kissel, T. Prospects for cationic polymers in gene and oligonucleotide therapy against cancer. Adv. Drug Deliv. Rev. 2002, 54, 715–758.
Miyata, K.; Kakizawa, Y.; Nishiyama, N.; Yamasaki, Y.; Watanabe, T.; Kohara, M.; Kataoka, K. Freeze-dried formulations for in vivo gene delivery of PEGylated polyplex micelles with disulfide crosslinked cores to the liver. J. Control. Release 2005, 109, 15–23.
Sun, P.; Huang, W.; Jin, M.; Wang, Q.; Fan, B.; Kang, L.; Gao, Z. Chitosan-based nanoparticles for survivin targeted siRNA delivery in breast tumor therapy and preventing its metastasis. Int. J. Nanomed. 2016, 11, 4931–4945.
Cai, X.; Yang, L. Q.; Huang, Y. F.; Peng, N. F.; Zhang, L. M.; Wu, Q.; Chen, R. F. Targeted and controlled release of indomethacin from a prodrug of amylose. In Proceedings of the 7th Asian-Pacific Conference on Medical and Biological Engineering, Berlin, Heidelberg, 2008, pp 29–31.
Gao, W.; Sha, B. Y.; Zou, W.; Liang, X.; Meng, X. Z.; Xu, H.; Tang, J.; Wu, D. C.; Xu, L. X.; Zhang, H. Cationic amylose-encapsulated bovine hemoglobin as a nanosized oxygen carrier. Biomaterials 2011, 32, 9425–9433.
Moghadam, S. H.; Wang, H. W.; Saddar El-Leithy, E.; Chebli, C.; Cartilier, L. Substituted amylose matrices for oral drug delivery. Biomed. Mater. 2007, 2, S71–S77.
Qiu, C.; Qin, Y.; Zhang, S. L.; Xiong, L.; Sun, Q. J. A comparative study of size-controlled worm-like amylopectin nanoparticles and spherical amylose nanoparticles: Their characteristics and the adsorption properties of polyphenols. Food Chem. 2016, 213, 579–587.
Pahimanolis, N.; Sorvari, A.; Luong, N. D.; Seppälä, J. Thermoresponsive xylan hydrogels via copper-catalyzed azide-alkyne cycloaddition. Carbohydr. Polym. 2014, 102, 637–644.
Ma, D.; Zhang, H. B.; Chen, Y. Y.; Lin, J. T.; Zhang, L. M. New cyclodextrin derivative containing poly(L-lysine) dendrons for gene and drug co-delivery. J. Colloid Interface Sci. 2013, 405, 305–311.
Pang, J. D.; Zhuang, B. X.; Mai, K. J.; Chen, R. F.; Wang, J.; Zhang, L. M. Click modification of helical amylose by poly(L-lysine) dendrons for non-viral gene delivery. Mater. Sci. Eng. C 2015, 49, 485–492.
Wang, F.; Ke, Z. F.; Wang, R.; Wang, Y. F.; Huang, L. L.; Wang, L. T. Astrocyte elevated gene-1 (AEG-1) promotes osteosarcoma cell invasion through the JNK/c-Jun/MMP-2 pathway. Biochem. Biophys. Res. Common. 2014, 452, 933–939.
Yu, C. P.; Chen, K.; Zheng, H. Q.; Guo, X. Z.; Jia, W. H.; Li, M. Z.; Zeng, M. S.; Li, J.; Song, L. B. Overexpression of astrocyte elevated gene-1 (AEG-1) is associated with esophageal squamous cell carcinoma (ESCC) progression and pathogenesis. Carcinogenesis 2009, 30, 894–901.
Emdad, L.; Sarkar, D.; Su, Z. Z.; Randolph, A.; Boukerche, H.; Valerie, K.; Fisher, P. B. Activation of the nuclear factor ?B pathway by astrocyte elevated gene-1: Implications for tumor progression and metastasis. Cancer Res. 2006, 66, 1509–1516.
Yoo, B. K.; Emdad, L.; Su, Z. Z.; Villanueva, A.; Chiang, D. Y.; Mukhopadhyay, N. D.; Mills, A. S.; Waxman, S.; Fisher, R. A.; Llovet, J. M. et al. Astrocyte elevated gene-1 regulates hepatocellular carcinoma development and progression. J. Clin. Invest. 2009, 119, 465–477.
Kawata, E.; Ashihara, E.; Kimura, S.; Takenaka, K.; Sato, K.; Tanaka, R.; Yokota, A.; Kamitsuji, Y.; Takeuchi, M.; Kuroda, J. et al. Administration of PLK-1 small interfering RNA with atelocollagen prevents the growth of liver metastases of lung cancer. Mol. Cancer Ther. 2008, 7, 2904–2912.
Huh, M. S.; Lee, E. J.; Koo, H.; Yhee, J. Y.; Oh, K. S.; Son, S.; Lee, S.; Kim, S. H.; Kwon, I. C.; Kim, K. Polysaccharidebased nanoparticles for gene delivery. Topics Curr. Chem. 2017, 375, 31.
Raemdonck, K.; Martens, T. F.; Braeckmans, K.; Demeester, J.; De Smedt, S. C. Polysaccharide-based nucleic acid nanoformulations. Adv. Drug Deliv. Rev. 2013, 65, 1123–1147.
Rejman, J.; Oberle, V.; Zuhorn, I. S.; Hoekstra, D. Sizedependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem. J. 2004, 377, 159–169.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 81402221 and 51273216), the Research Fund for the Doctoral Program of Higher Education of China (No. 20130171120077), the Science and Technology Program of Guangzhou, China (No. 201707010108), the Guangdong Innovative Research Team Program (No. 2009010057), and the Science and Technology Planning Project of Guangzhou (No. 201610010006), the Science and Technology Planning project of Guangdong Province (No. 20153900042020319) and Natural Science Foundation of Guangdong Province (No. 2016A030313819).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
12274_2018_1965_MOESM1_ESM.pdf
Inhibition of osteosarcoma growth and metastasis using a polysaccharide derivative of Amy-g-PLLD for the delivery of AEG-1 siRNA
Rights and permissions
About this article
Cite this article
Wang, F., Pang, J., Huang, L. et al. Inhibition of osteosarcoma growth and metastasis using a polysaccharide derivative of Amy-g-PLLD for the delivery of AEG-1 siRNA. Nano Res. 11, 3886–3898 (2018). https://doi.org/10.1007/s12274-018-1965-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-018-1965-4