Abstract
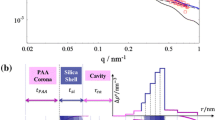
Herein, we fabricate hollow silica nanoparticles with exceptionally narrow size distributions that inherently possess two distinct length scales—tens of nanometers with regards to the shell thickness, and hundreds of nanometers in regards to the total diameter. We characterize these structures using dynamic and static light scattering (DLS and SLS), small angle X-ray scattering (SAXS), and transmission electron microscopy (TEM), and we demonstrate quantitative agreement among all methods. The ratio between the radius of gyration (SLS) and hydrodynamic radius (DLS) in these particles equals almost unity, corresponding to ideal capsule behavior. We are able to resolve up to 20 diffraction orders of the hollow sphere form factor in SAXS, indicating a narrow size distribution. Data from light and X-ray scattering can be combined to a master curve covering a q-range of four orders of magnitude assessing all hierarchical length scales of the form factor. The measured SLS intensity profiles noticeably change when the scattering contrast between the interior and exterior is altered, whereas the SAXS intensity profiles do not show any significant change. Tight control of the aforementioned length scales in one simple and robust colloidal building block renders these particles suitable as future calibration standards.

Similar content being viewed by others
References
Choi, H.; Sofranko, A. C.; Dionysiou, D. D. Nanocrystalline TiO2 photocatalytic membranes with a hierarchical mesoporous multilayer structure: Synthesis, characterization, and multifunction. Adv. Funct. Mater. 2006, 16, 1067–1074.
Rhee do, K.; Jung, B.; Kim, Y. H.; Yeo, S. J.; Choi, S. J.; Rauf, A.; Han, S.; Yi, G. R.; Lee, D.; Yoo, P. J. Particlenested inverse opal structures as hierarchically structured large-scale membranes with tunable separation properties. ACS Appl. Mater. Interfaces 2014, 6, 9950–9954.
Su, B.-L.; Sanchez, C.; Yang, X.-Y. Hierarchically Structured Porous Materials: From Nanoscience to Catalysis, Separation, Optics, Energy, and Life Science; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, 2011.
Cho, C.-Y.; Moon, J. H. Hierarchical twin-scale inverse opal TiO2 electrodes for dye-sensitized solar cells. Langmuir 2012, 28, 9372–9377.
Wang, D. Y.; Möhwald, H. Template-directed colloidal selfassembly–the route to 'top-down' nanochemical engineering. J. Mater. Chem. 2004, 14, 459–468.
von Freymann, G.; Kitaev, V.; Lotsch, B. V.; Ozin, G. A. Bottom-up assembly of photonic crystals. Chem. Soc. Rev. 2013, 42, 2528–2554.
Vogel, N.; Retsch, M.; Fustin, C. A.; Del Campo, A.; Jonas, U. Advances in colloidal assembly: The design of structure and hierarchy in two and three dimensions. Chem. Rev. 2015, 115, 6265–6311.
Gröschel, A. H.; Walther, A.; Löbling, T. I.; Schacher, F. H.; Schmalz, H.; Müller, A. H. E. Guided hierarchical coassembly of soft patchy nanoparticles. Nature 2013, 503, 247–251.
Cosgrove, T. Colloid Science: Principles, Methods and Applications, 2nd ed.; Wiley-Blackwell: Oxford, 2010.
Chen, Z. H.; Kim, C.; Zeng, X. B.; Hwang, S. H.; Jang, J.; Ungar, G. Characterizing size and porosity of hollow nanoparticles: SAXS, SANS, TEM, DLS, and adsorption isotherms compared. Langmuir 2012, 28, 15350–15361.
Blanton, T. N.; Huang, T. C.; Toraya, H.; Hubbard, C. R.; Robie, S. B.; Louër, D.; Göbel, H. E.; Will, G.; Gilles, R.; Raftery, T. JCPDS—International Centre for Diffraction Data round robin study of silver behenate. A possible low-angle X-ray diffraction calibration standard. Powder Diffr. 1995, 10, 91–95.
Huang, T. C.; Toraya, H.; Blanton, T. N.; Wu, Y. X-ray powder diffraction analysis of silver behenate, a possible low-angle diffraction standard. J. Appl. Crystallogr. 1993, 26, 180–184.
Nyam-Osor, M.; Soloviov, D. V.; Yu, S. K.; Zhigunov, A.; Rogachev, A. V.; Ivankov, O. I.; Erhan, R. V.; Kuklin, A. I. Silver behenate and silver stearate powders for calibration of SAS instruments. J. Phys.: Conf. Ser. 2012, 351, 012024.
Orgel, J. P. R. O.; Irving, T. C.; Miller, A.; Wess, T. J. Microfibrillar structure of type I collagen in situ. Proc. Natl. Acad. Sci. USA 2006, 103, 9001–9005.
Orgel, J. P. R. O.; Miller, A.; Irving, T. C.; Fischetti, R. F.; Hammersley, A. P.; Wess, T. J. The in situ supermolecular structure of type I collagen. Structure 2001, 9, 1061–1069.
Patel, I. S.; Schmidt, P. W. Small-angle X-ray scattering determination of the electron density of the particles in a colloidal suspension. J. Appl. Crystallogr. 1971, 4, 50–55.
Russell, T. P. An absolute intensity standard for small-angle X-ray scattering measured with position-sensitive detectors. J. Appl. Crystallogr. 1983, 16, 473–478.
Perret, R.; Ruland, W. Glassy carbon as standard for the normalization of small-angle scattering intensities. J. Appl. Crystallogr. 1972, 5, 116–119.
Russell, T. P.; Lin, J. S.; Spooner, S.; Wignall, G. D. Intercalibration of small-angle X-ray and neutron scattering data. J. Appl. Crystallogr. 1988, 21, 629–638.
Dreiss, C. A.; Jack, K. S.; Parker, A. P. On the absolute calibration of bench-top small-angle X-ray scattering instruments: A comparison of different standard methods. J. Appl. Crystallogr. 2006, 39, 32–38.
Chen, M.; Ye, C. Y.; Zhou, S. X.; Wu, L. M. Recent advances in applications and performance of inorganic hollow spheres in devices. Adv. Mater. 2013, 25, 5343–5351.
Kohlbrecher, J. SASfit: A Program for Fitting Simple Structural Models to Small Angle Scattering Data. Paul Scherrer Institute, Laboratory for Neutron Scattering: Villigen, Switzerland, 2014.
Ruckdeschel, P.; Kemnitzer, T. W.; Nutz, F. A.; Senker, J.; Retsch, M. Hollow silica sphere colloidal crystals: Insights into calcination dependent thermal transport. Nanoscale 2015, 7, 10059–10070.
Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69.
Förster, S.; Apostol, L.; Bras, W. Scatter: Software for the analysis of nano- and mesoscale small-angle scattering. J. Appl. Crystallogr. 2010, 43, 639–646.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Ruckdeschel, P., Dulle, M., Honold, T. et al. Monodisperse hollow silica spheres: An in-depth scattering analysis. Nano Res. 9, 1366–1376 (2016). https://doi.org/10.1007/s12274-016-1032-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-016-1032-y