Abstract
A novel coronavirus, later named SARS-CoV-2, was first reported in China in December 2019 and subsequently widely identified in the United States, Japan, South Korea, France, India, and other countries. The disease caused by SARS-CoV-2 infection was called COVID-19. The high fatality and morbidity rates of COVID-19 make it the third largest global epidemic in this century. However, there are currently no approved antiviral drugs for the COVID-19 treatment. Recently, two old antimalarial drugs, hydroxychloroquine and chloroquine, have been found to exert anti-SARS-CoV-2 effects both in vitro and in vivo. Preliminary clinical evidence suggests these drugs may have an effect on the treatment of COVID-19. Herein, we review the pharmacokinetics characteristics and antiviral effects of these drugs, in addition to their side effects and clinical evidence of their use for the COVID-19 treatment.
Similar content being viewed by others
Introduction
In December 2019, unexplained pneumonia cases were reported in Wuhan, China, which was later confirmed to be caused by a novel coronavirus identified as SARS-CoV-2. The clinical symptoms caused by this novel coronavirus were named as coronavirus disease 2019 (COVID-19) by World Health Organization (WHO). SARS-CoV-2 is a β-coronavirus and its genomic sequence is 96.2% identical to that of Bat-SARSs-CoV RaTG13 and 79.5% identical to that of SARS-CoV. Based on the sequencing results and evolutionary analysis of the viral genome, bats are suspected to be the natural hosts of SARS-CoV-2, while the intermediate host is unknown. Previous studies have shown that SARS-CoV-2, like SARS-CoV, can infect human cells that express angiotensin-converting enzyme 2 (ACE2) (Guo et al. 2020).
The most common symptoms of COVID-19 patients include fever, cough, fatigue, and myalgia. However, the disease can evolve in some cases and patients begin to experience dyspnea, shock, multiple organ damage and may even die (Huang et al. 2020; Wang et al. 2020a; Zhang et al. 2020). COVID-19 is reported to be transmitted from person to person through respiratory droplets or by direct contact with infected patients. Although virus particles have been detected in feces, there is no clear evidence that the virus can be transmitted through the digestive tract (Adhikari et al. 2020). Studies estimate that the basic reproduction number (R0) of SARS-CoV-2 is about 2.2, but it can vary from 1.4 to 6.5 (Adhikari et al. 2020; Guo et al. 2020; Imai et al. 2020; Shen et al. 2020). As of April 30, 3,172,185 cases have been confirmed worldwide, and 228,720 (7.21%) have died (WHO 2020). According to WHO, more than 200 countries and regions have been affected by pneumonia induced by SARS-Cov-2 (WHO 2020).
A variety of therapeutic strategies have been tried for the COVID-19 treatment, including antiviral drugs and antibacterial drugs, glucocorticoids, traditional Chinese medicine, among others., However, no drugs has been approved for the COVID-19 treatment so far. Recently, two antimalarial drugs, chloroquine (CQ) and hydroxychloroquine (HCQ), have been shown to have potential anti-SARS-CoV-2 activity and have achieved promising results in clinical treatments (Gautret et al. 2020a; Mitjà and Clotet 2020; Wang et al. 2020b). However, these drugs have certain limitations and toxicity, especially on the heart and eyes (Motarjemizadeh et al. 2015; Chatre et al. 2018). Herein, we discuss the pharmacological properties of CQ and HCQ, and their efficacy and side effects in the treatment of COVID-19.
Pharmacokinetics
Chloroquine sulfates and phosphates have been commercialized as antimalarial drugs, and are referred to below as chloroquine (CQ). Hydroxychloroquine (HCQ), a CQ derivative, is also used as an antimalarial drug, but is currently widely used in autoimmune diseases, such as lupus and rheumatoid arthritis. Both belong to 4-aminoquinoline derivatives group and share many similar pharmacological properties (Touret and de Lamballerie 2020). The gastrointestinal tract absorbs 75 to 100% of the CQ administered orally and the plasma concentration reaches its peak in 2 to 4.5 h. CQ binds to plasma proteins in the plasma and slowly reaches steady state concentrations. Volunteers who took 150 mg of CQ orally, had plasma concentration of CQ and HCQ after 4 h of ingestion on the 5th day of treatment of 167.14 and 34.48 μg/L, respectively. The plasma CQ elimination half-life is about 32 days. CQ is excreted mainly by the kidneys (about 40–50%) and the rest is excreted by feces (Furst 1996).
The bioavailability of HCQ can reach 50% after oral administration. The highest blood concentration is reached 1 to 2 h after taking the medication. About 55% of the drug binds to the plasma components in the blood. The concentration in the liver, spleen, kidney and lung is 200 to 700 times higher than the plasma concentration and in the brain and spinal cord tissue it is 10 to 30 higher. The HCQ metabolic conversion in the body occurs in the liver and its main metabolite is desethylchloroquine. The half-life of HCQ is between 40 and 50 days and the blood clearance rate are low, around 96 ml/min (Furst 1996).
CQ and HCQ antiviral effects
CQ and HCQ were first used in the prevention and treatment of malaria, but have been reported to display anti-inflammatory, immunomodulatory, anti-infective, anti-thrombotic and metabolic effects (Plantone and Koudriavtseva 2018). In vitro, CQ completely inhibits the Plasmodium vivax (P. vivax) development in the 40–1280 nM concentration range (Tasanor et al. 2002). In vivo, at an effective dose of 250 mg/day, the effective plasma concentrations of CQ that killed P. vivax and P. falciparum parasites were 15 and 20 μg/L, respectively. CQ/HCQ plasma concentration use to treat rheumatoid arthritis is about 250–650 μg/L (Ducharme and Farinotti 1996). So far, CQ and HCQ have been used successfully to treat a variety of infectious, rheumatoid and other immune diseases (Al-Bari 2015). In addition, these drugs have antiviral effects which will be discussed below.
As early as 1969, CQ has been reported to present antiviral activity (Inglot 1969). In vitro experiments found that CQ and HCQ can inhibit the growth of a variety of viruses including SARS-coronavirus, human coronavirus OC43, enterovirus EV-A71, Zika Virus and Influenza A H5N1 (Keyaerts et al. 2004, 2009; Yan et al. 2013; Li et al. 2017; Tan et al. 2018).
Previous studies found that CQ and HCQ exerts antiviral effects through multiple mechanisms. Both compounds are soluble in water and are weakly alkaline. After entering the cell, they induce a pH increase in acidic organelles (including endosomes, lysosomes, and Golgi vesicles), thereby destroying them. Moreover, an increased intracellular pH has been shown to block viral infections (Legssyer et al. 2003). The low pH can trigger the virus invasion in the cell, either by fusion, penetration or uncoating. On the other hand, an increased intracellular pH will prevent the viral infection process. The uncoating of influenza B virus process is hindered when the lysosomal pH is higher than the critical value required to induce fusion between the viral envelope and the lysosomal membrane (Shibata et al. 1983; Bishop 1998). In addition, an increased pH in the endoplasmic reticulum and Golgi can affect the post-translational modification of viral envelope glycoproteins, thereby decreasing the viral maturation rate (Randolph et al. 1990).
In addition to the effects on cell pH, CQ can affect viral replication by inhibiting viral gene expression. In vitro and in vivo experiments have found that CQ can change the glycosylation pattern of HIV-1 envelope glycoprotein GP120 and inhibit the replication of the HIV replication in CD4+ T cells (Naarding et al. 2007). Furthermore, CQ can also prevent the virus cell infection by interfering with the glycosylation of SARS-CoV receptors (Vincent et al. 2005). As SARS-CoV-2 and SARS-CoV enter cells via ACE2, a previous study has measured the ability of CQ and HCQ to inhibit SARS-CoV-2 infection and found that both drugs significantly inhibited SARS-CoV-2 infection in vitro (Liu et al. 2020; Wang et al. 2020b). Researchers measured the effect of CQ on SARS-CoV-2 in Vero E6 cells and obtained a half-maximal effective concentration (EC50) of 1.13 μM; a half-cytotoxic concentration (CC50) greater than 100 μM and a selectivity index (SI) greater than 88.50 (Wang et al. 2020b). In addition, another study found that the viral inhibitory CQ concentration range is 0.5–10 mol/L and that these concentrations have been shown to be achievable in plasma during clinical treatment of malaria (1.6 to 12.5 μmol/L). (Ducharme and Farinotti 1996).
Moreover, CQ has been reported to inhibits autophagy and thus interfere with virus infection and replication. In vivo data found that CQ can effectively inhibit autophagy in mouse lung induced by avian influenza H5N1 and reduce alveolar epithelial damage (Yan et al. 2013).
CQ and HCQ anti-inflammatory effects
In addition to direct antiviral activity, HCQ and CQ are effective anti-inflammatory drugs. They can significantly reduce the cytokines production, such as IL-1, IL-2, IL-6 or IL-18, TNF-α and INF-γ, which have been shown to increase significantly in COVID-19 patients and are closely related to acute respiratory distress syndrome (ARDS) and adverse outcomes(Al-Bari 2015; Liu et al. 2020; Wu et al. 2020). A clinical investigation found that high concentrations of cytokines were detected in the plasma of critically ill COVID-19 patients, revealing that a cytokine storm is related to the disease severity (Huang et al. 2020). These data suggest that anti-inflammatory effects may be one of the pathways used by HCQ and CQ to decrease SARS-CoV-2 infection.
CQ and HCQ clinical evidence in the COVID-19 treatment
CQ and HCQ are recommended for the treatment of COVID-19 in many regions and countries (Mediterranee-infection Hospital 2020). In the sixth edition of China’s Novel Coronavirus Pneumonia Diagnosis and Treatment Plan issued by the Chinese National Health Commission, CQ was recommended as one of the COVID-19 treatments options. It recommends a 500 mg oral dose of chloroquine phosphate in adults (contains 300 mg of chloroquine), twice daily, for no more than 10 days. In the updated seventh edition, the chloroquine dosage, the target population and the course of treatment were clearly defined. The dose is adjusted according to the patient’s body weight. When the body weight is > 50 kg, a 500 mg oral dose of chloroquine phosphate is recommended twice daily. When the body weight is < 50 kg, the recommended dose is 500 mg, twice daily for the first 2 days. For the next 5 days, the medication frequency is adjusted to once a day. The recommended course of treatment is 1 week.
In a French clinical study that included 19 COVID-19 patients, a 600 mg daily dose of HCQ was significantly associated with a decrease or disappearance of viral load in patients (Gautret et al. 2020a). On March 20, that same team published a study containing a larger sample of 80 COVID-19 patients. The results showed that, with the exception of the death of one 86-year-old patient and one 74-year-old patient who were out of the intensive care unit, all patients who received their treatment regimen showed clinical improvement and rapid decline in nasopharyngeal viral load. On day 7, 83% of the patients tested negative for SARS-CoV-2 genome and on day 8, that rate rose to 93% (Gautret 2020b). This same study also found that the combination of HCQ and azithromycin was able to improve the therapeutic effect (Gautret 2020b). Based on the results of this study, the U.S. FDA issued an emergency use authorization (EUA) to approve the use of CQ and HCQ for the treatment of COVID-19 hospitalized patients with on March 28 (FDA 2020).
A meta-analysis, containing 6 completed clinical trials and 23 ongoing in China, strongly suggested that CQ could be a potential treatment for COVID-19 (Cortegiani et al. 2020). A study by the Shanghai Public Health Center showed that the COVID-19 patients who received hydroxychloroquine sulfate had better prognosis (Chen et al. 2020a). Another randomized controlled trial on HCQ was conducted in Wuhan, China. This study randomly divided 62 COVID-19 patients into test and the placebo groups, which were supplemented with HCQ (400 mg/day) or placebo for 5 days, respectively. Then, it was found that the proportion of patients with symptomatic improvement in the HCQ treatment group (80.6%, 25 of 31 cases) was significantly higher than that of the control group (54.8%, 17 of 31 cases) (Chen et al. 2020b). Taken together, these clinical studies indicate that CQ and HCQ may be one of the potential treatment options for COVID-19.
Arguments for using CQ and HCQ in the COVID-19 treatment
Despite the above in vitro and in vivo evidence reported above, HCQ and CQ clinical application in the COVID-19 treatment is still very controversial. For example, the leaders of many health and research agencies were opposed to the FDA decision on March 28. They argued that this decision destroyed the scientific authority of FDA and that it has a clear meaning of just enthusiastically support politicians (Piller 2020).
The European Medicines Agency (EMA) allows the use of these drugs for clinical trials only and recommends that their urgent use be in accordance with the policies of each country (EMA 2020). The EMA noted in its statement that CQ and HCQ are being studied worldwide because of their potential to treat COVID-19. However, the efficacy of CQ/HCQ treatment in COVID-19 has not been shown in well-designed clinical studies. Both CQ and HCQ may have serious side effects, especially at high doses or when combined with other drugs. They can only be used in clinical trials or nationally approved programmes (EMA 2020). In fact, a small sample study from France also showed that the combination of HCQ and azithromycin combination does not provide sufficient evidence to quickly eliminate the virus or provide clinical benefits in critically ill COVID-19 patients (Molina et al. 2020).
In addition, on April 3, the International Society of Antimicrobial Chemotherapy (ISAC) issued a statement stating that the article published by its sponsored journal demonstrating that “hydroxychloroquine is an effective medicine for COVID-19” did not meet the expected standard of ISAC (Voss 2020). In particular, the lack of detailed explanations of the criteria for patients inclusion and classification makes the study conclusion weak (Voss 2020).
Although some studies have shown that CQ/HCQ has a therapeutic effect on COVID-19, other studies believe that it is difficult to compare the drug efficacy in studies using a primary end point of viral negative with studies using severe rate (Chen et al. 2020a). They argue believed that it is necessary to conduct follow-up surveys to determine the most appropriate population and end point events and to fully consider the feasibility of the clinical trials, such as the study sample size (Chen et al. 2020a).
However, an international vote of 6227 doctors from 30 countries and regions released by the global medical voting company Sermo found that of the 15 treatment alternatives, 37% of doctors rated HCQ as “the most effective treatment for COVID-19” (Sermo.com 2020). Therefore, evidence from large multi-center randomized controlled clinical trials is required to confirm these results and to determine the duration and dose to achieve individualized medication. Clinical trials should find a more appropriate populational size or endpoint indicator that is most appropriate to evaluate the CQ/HCQ (and all other drugs) effect on clinical feasibility and the assessment of reduced the mortality rates in severe or critical illness patients (Chen et al. 2020a). It is important to note that some COVID-19 patients have several comorbidities, and some are pregnant women and children. Thus, a special attention should be given to these patients when considering CQ and HCQ treatment.
Toxicology
Both HCQ and CQ have good safety records and display mild side effects. Gastrointestinal reactions, such as vomiting and diarrhea are the most common side effects of these drugs. Other side effects include dizziness, headache, dazzling, tinnitus, disturbances of taste and smell, seizures, psychosis, and irritability. These side effects are usually mild and can disappear after stopping treatment (Al-Bari 2015; Srinivasa et al. 2017).
On the other hand, patients who took these drugs for long periods experienced toxic effects, such as retinopathy, circular defects (or bull’s eye maculopathy), and retinal diameter defects, which are generally irreversible, mainly due to drug accumulation in the eye (Schrezenmeier and Dörner 2020). Among toxic effects, retinopathy is the more serious clinical problem that can cause irreversible damage to vision, and even blindness. The American Academy of Ophthalmology determined the risk factors for retinopathy due to HCQ use, which include a time of use greater than 5 years, a high daily drug dosage (HCQ > 5.0 mg/kg lean body mass; CQ > 2.3 mg/kg lean body mass), renal or liver disease patient, high body fat levels, use of concomitant drugs similar to tamoxifen, macular disease and age over 60 years (Marmor et al. 2016). Remarkably, the daily dose and duration of use are the main risk factors. When the HCQ daily dose exceeds 20 mg/kg, the incidence of retinopathy is a 25 to 40% within 1 to 2 years (Marmor et al. 2016). Although the use duration of HCQ and CQ in COVID-19 patients is generally very short, it is still necessary to be aware of their retinal toxicity. Retinal degeneration caused by HCQ and CQ can continue to develop even after treatment is stopped.
The CQ and HCQ side effects in the heart are mainly conduction disorders and cardiomyopathy, which are usually irreversible and fatal (Frisk-Holmberg et al. 1983; Tönnesmann et al. 2013). The cardiotoxicity of these drugs may be related to the their long-term use, which causes acquired lysosomal accumulation disorder. This eventually leads to drug-induced cardiomyopathy, or in the case of excessive doses ingestion, acute poisoning (Yogasundaram et al. 2014). There is a case report of an elderly woman with lupus patient who used HCQ for a long time was diagnosed with refractory ventricular arrhythmia due to a prolonged QT interval. After excluding other causes, she was diagnosed with cardiac conduction abnormalities caused by chronic HCQ poisoning (Chen et al. 2006).
Because CQ and HCQ affect the cardiac conduction system, their use should be avoided in combination with other drugs that block cardiac conduction to prevent fatal arrhythmia. Among them, digitalis drugs (digoxin, desacetylgoxin, digitoxin, and trichoside K), antiarrhythmic drugs class Ia (quinidine and procainamide), antiarrhythmic drugs class III (amiodarone, sotalol, ibutilide, and dronedarone), benpredil, hydrochlorothiazide, and indapamide. In addition, the CQ/HCQ combination with antibiotics, such as quinolones and macrolides, should be prohibited to avoid the risk of promoting the QT interval prolongation and leading to tip torsion. In addition, it is worth mentioning that the interval between the therapeutic and toxic doses of CQ and HCQ is narrow, and acute CQ poisoning is associated with a potential life-threatening cardiovascular disease (Touret and de Lamballerie 2020).
Regarding the nervous system, currently there is no experimental evidence that CQ, HCQ and their metabolites can affect its conduction properties. It is only known that quinine is neurotoxic to dopaminergic neurons in the limbic system (Zou et al. 2018). Further studies are needed to investigate the CQ and HCQ effects on the central and peripheral nervous systems. Therefore, the CQ and HCQ use should follow strict rules and self-medication is not recommended. Moreover, for patients using these drugs, ECG and echocardiographic monitoring should be performed regularly to early detect potential cardiac toxicity early (Yogasundaram et al. 2014).
Over the years, researchers have measured the correlation between CQ/HCQ and toxicity. Their toxicological properties are concentration-dependent. The concentration that causes mild side effects, such as dizziness, diplopia, and fatigue, is about 0.5 to 1.2 × 10–6 mol/L. According to previous reports, about 80% of patients with side effects have CQ/HCQ plasma concentration higher than 2.5 × 10–6 mol/L. On the other hand, no toxic reaction has been reported when their plasma concentration is below 1.25 × 10–6 mol/L. Finally, the critical plasma concentration of cardiovascular toxicity caused by CQ is about 1000 μg/L (3 × 10−6 mol/L) (Ducharme and Farinotti 1996).
Conclusion and suggestions
In summary, HCQ and CQ were found to have exert anti-SARS-CoV-2 effects both in vitro and in vivo, and represent potential treatment options for COVID-19 (Fig. 1). However, the clinical evidence of their effects is from some single-center clinical trials. Evidence from multi-center clinical trials is still lacking. In accordance with the pharmacological and toxicological effects of CQ and HCQ discussed here, the authors of this review recommend the following: (1) Multicenter randomized controlled clinical trials are needed to clarify the CQ and HCQ efficiency and safety in the COVID-19 treatment; (2) Considering their ocular, cardiac and neuro toxicities, CQ and HCQ should not be recommended as preventive drugs for the COVID-19 pandemic; (3) Follow-up of patients who received CQ or HCQ treatment is necessary to access their long-term effects and side effects; (4) The CQ and HCQ dosage and their combination regimen with other drugs in clinical trials should be appropriately adjusted.
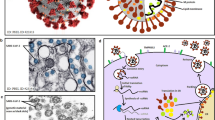
Anti-SARS-CoV-2 effects of HCQ and CQ exert both in vitro and in vivo. Descriptive graphic shown the process of SARS-CoV-2 infection and replication, and the anti-SARS-CoV-2 effects of HCQ and CQ in it. (1) CQ and HCQ can glycosylate SARS-CoV-2 itself and its receptor. (2) CQ and HCQ can inhibit cell autophagy, which was involve in virus infection process. (3) CQ and HCQ can directly inhibit the replication of RNA viruses. (4) CQ and HCQ can directly inhibit the expression of SARS-CoV viral gene. (5) After CQ and HCQ directly enter the cell body, the intracellular pH rises and inhibits a series of viral replication and recombination processes
References
Adhikari SP, Meng S, Wu YJ, Mao YP, Ye RX, Wang QZ, Sun C, Sylvia S, Rozelle S, Raat H, Zhou H (2020) Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty 9:29. https://doi.org/10.1186/s40249-020-00646-x
Al-Bari MA (2015) Chloroquine analogues in drug discovery: new directions of uses, mechanisms of actions and toxic manifestations from malaria to multifarious diseases. J Antimicrob Chemother 70:1608–1621. https://doi.org/10.1093/jac/dkv018
Bishop NE (1998) Examination of potential inhibitors of hepatitis A virus uncoating. Intervirology 41:261–271. https://doi.org/10.1159/000024948
Chatre C, Roubille F, Vernhet H, Jorgensen C, Pers YM (2018) Cardiac complications attributed to chloroquine and hydroxychloroquine: a systematic review of the literature. Drug Saf 41:919–931. https://doi.org/10.1007/s40264-018-0689-4
Chen CY, Wang FL, Lin CC (2006) Chronic hydroxychloroquine use associated with QT prolongation and refractory ventricular arrhythmia. Clin Toxicol (Phila) 44:173–175. https://doi.org/10.1080/15563650500514558
Chen J, Liu D, Liu L, Liu P, Xu Q, Xia L, Ling Y, Huang D, Song S, Zhang D (2020a) A pilot study of hydroxychloroquine in treatment of patients with moderate COVID-19. Zhejiang Da Xue Xue Bao Yi Xue Ban 49:215–219
Chen Z, Hu J, Zhang Z, Jiang S, Han S, Yan D, Zhuang R, Hu B, Zhang Z (2020b) Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. MedRxiv. https://doi.org/10.1101/2020.03.22.2004075
Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S (2020) A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care 57:279–283. https://doi.org/10.1016/j.jcrc.2020.03.005
Ducharme J, Farinotti R (1996) Clinical pharmacokinetics and metabolism of chloroquine. Focus on recent advancements. Clin Pharmacokinet 31:257–274. https://doi.org/10.2165/00003088-199631040-00003
EMA(European Medicines Agency) (2020) COVID-19: chloroquine and hydroxychloroquine only be used in clinical trials or emergency use programmes. Available at European Medicines Agency document Website https://www.ema.europa.eu/en/documents/press-release/covid-19-chloroquine-hydroxychloroquine-only-be-used-clinical-trials-emergency-use-programmes_en.pdf. Accessed 1 Apr 2020
FDA(US Food & Drug Administration) (2020) Re: Request for Emergency Use Authorization For Use of Chloroquine Phoslhate or Hydroxychloroquine Sulfate Supplied From the Strategic National Stockpile for Treatment of 2019 Covrnavirus Disease. US Food & Drug Administration official Website. Available at https://www.fda.gov/media/136534/download. Accessed 29 Mar 2020
Frisk-Holmberg M, Bergqvist Y, Englund U (1983) Chloroquine intoxication. Br J Clin Pharmacol 15:502–503. https://doi.org/10.1111/j.1365-2125.1983.tb01540.x
Furst DE (1996) Pharmacokinetics of hydroxychloroquine and chloroquine during treatment of rheumatic diseases. Lupus 5:S11–S15
Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Dupont HT, Honore S, Colson P, Chabriere E, La Scola B, Rolain JM, Brouqui P, Raoult D (2020a) Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. https://doi.org/10.1016/j.ijantimicag.2020.105949
Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Sevestre J, Mailhe M, Doudier B, Aubry C, Amrane S, Seng P, Hocquart M, Eldin C, Finance J, Vieira VE, Tissot-Dupont HT, Honoré S, Stein A, Million M, Colson P, La Scola B, Veit V, Jacquier A, Deharo JC, Drancourt M, Fournier PE, Rolain JM, Brouqui P, Raoult D (2020b) Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: an observational study. Travel Med Infect Dis 34:101663. https://doi.org/10.1016/j.tmaid.2020.101663
Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, Tan KS, Wang DY, Yan Y (2020) The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak–an update on the status. Mil Med Res 7:10–11. https://doi.org/10.1186/s40779-020-00240-0
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet 395:497–506. https://doi.org/10.1016/S0140-6736(20)30183-5
Imai N, Cori A, Dorigatti I, Baguelin M, Donnelly CA, Riley S, Ferguson NM (2020) Report 3: transmissibility of 2019-nCoV. Feminine-Perspective Magazine Web. Available at https://fpmag.net/wp-content/uploads/2020/01/Imperial-2019-nCoV-transmissibility.pdf. Accessed 29 Mar 2020
Inglot AD (1969) Comparison of the antiviral activity in vitro of some non-steroidal anti-inflammatory drugs. J Gen Virol 4:203–214. https://doi.org/10.1099/0022-1317-4-2-203
Keyaerts E, Vijgen L, Maes P, Neyts J, Van Ranst M (2004) In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun 323:264–268. https://doi.org/10.1016/j.bbrc.2004.08.085
Keyaerts E, Li S, Vijgen L, Rysman E, Verbeeck J, Van Ranst M, Maes P (2009) Antiviral activity of chloroquine against human coronavirus OC43 infection in newborn mice. Antimicrob Agents Chemother 53:3416–3421. https://doi.org/10.1128/AAC.01509-08
Legssyer R, Josse C, Piette J, Ward RJ, Crichton RR (2003) Changes in function of iron-loaded alveolar macrophages after in vivo administration of desferrioxamine and/or chloroquine. J Inorg Biochem 94:36–42. https://doi.org/10.1016/s0162-0134(02)00633-5
Li C, Zhu X, Ji X, Quanquin N, Deng YQ, Tian M, Aliyari R, Zuo X, Yuan L, Afridi SK, Li XF, Jung JU, Nielsen-Saines K, Qin FX, Qin CF, Xu Z, Cheng G (2017) Chloroquine, a FDA-approved drug, prevents Zika Virus infection and its associated congenital microcephaly in mice. EBioMedicine 24:189–194. https://doi.org/10.1016/j.ebiom.2017.09.034
Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, Li Y, Hu Z, Zhong W, Wang M (2020) Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov 6:16. https://doi.org/10.1038/s41421-020-0156-0
Marmor MF, Kellner U, Lai TY, Melles RB, Mieler WF, American Academy of Ophthalmology (2016) Recommendations on screening for chloroquine and hydroxychloroquine etinopathy (2016 Revision). Ophthalmology 123:1386–1394. https://doi.org/10.1016/j.ophtha.2016.01.058
Mediterranee-infection Hospital (2020) Countries and regions where hydroxychloroquine and chloroquine are recommended to fight coronavirus. Available at Mediterranee-infection Hospital Website https://www.mediterranee-infection.com/coronavirus-pays-ou-lhydroxychloroquine-est-recommandee. Accessed 28 Mar 2020
Mitjà O, Clotet B (2020) Use of antiviral drugs to reduce COVID-19 transmission. Lancet Glob Health 8:e639–e640. https://doi.org/10.1016/S2214-109X(20)30114-5
Molina JM, Delaugerre C, Le Goff J, Mela-Lima B, Ponscarme D, Goldwirt L, de Castro N (2020) No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect 50:384. https://doi.org/10.1016/j.medmal.2020.03.006
Motarjemizadeh Q, Aidenloo NS, Abbaszadeh M (2015) Detection of hydroxychloroquine retinal toxicity by automated perimetry in 60 rheumatoid arthritis patients with normal fundoscopic findings. Glob J Health Sci 8:59–64. https://doi.org/10.5539/gjhs.v8n3p59
Naarding MA, Baan E, Pollakis G, Paxton WA (2007) Effect of chloroquine on reducing HIV-1 replication in vitro and the DC-SIGN mediated transfer of virus to CD4+ T-lymphocytes. Retrovirology 4:6. https://doi.org/10.1186/1742-4690-4-6
Piller C (2020) Former FDA leaders decry emergency authorization of malaria drugs for coronavirus. Science Magazine NewWeb. Available at https://www.sciencemag.org/news/2020/04/former-fda-leaders-decry-emergency-authorization-malaria-drugs-coronavirus. Accessed 7 Apr 2020
Plantone D, Koudriavtseva T (2018) Current and future use of chloroquine and hydroxychloroquine in infectious, immune, neoplastic, and neurological diseases: a mini-review. Clin Drug Investig 38:653–671. https://doi.org/10.1007/s40261-018-0656-y
Randolph VB, Winkler G, Stollar V (1990) Acidotropic amines inhibit proteolytic processing of flavivirus prM protein. Virology 174:450–458. https://doi.org/10.1016/0042-6822(90)90099-d
Schrezenmeier E, Dörner T (2020) Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol 16:155–166. https://doi.org/10.1038/s41584-020-0372-x
Sermo.Com (2020) Breaking Results: Sermo’s COVID-19 Real Time Barometer Study. Available at Sermo’s COVID-19-Barometer Web https://public-cdn.sermo.com/covid19/c8/be4e/4edbd4/dbd4ba4ac5a3b3d9a479f99cc5/wave-i-sermo-covid-19-global-analysis-final.pdf. Accessed 27 Mar 2020.
Shen M, Peng Z, Xiao Y, Zhang L (2020) Modelling the epidemic trend of the 2019 novel coronavirus outbreak in China. Biorxiv. https://doi.org/10.1101/2020.01.23.916726
Shibata M, Aoki H, Tsurumi T, Sugiura Y, Nishiyama Y, Suzuki S, Maeno K (1983) Mechanism of uncoating of influenza B virus in MDCK cells: action of chloroquine. J Gen Virol 64:1149–1156. https://doi.org/10.1099/0022-1317-64-5-1149
Srinivasa A, Tosounidou S, Gordon C (2017) Increased Incidence of Gastrointestinal Side Effects in Patients Taking Hydroxychloroquine: A Brand-related Issue? J Rheumatol 44:398. https://doi.org/10.3899/jrheum.161063
Tan YW, Yam WK, Sun J, Chu JJH (2018) An evaluation of Chloroquine as a broad-acting antiviral against Hand, Foot and Mouth Disease. Antiviral Res 149:143–149. https://doi.org/10.1016/j.antiviral.2017.11.017
Tasanor O, Noedl H, Na-Bangchang K, Congpuong K, Sirichaisinthop J, Wernsdorfer WH (2002) An in vitro system for assessing the sensitivity of Plasmodium vivax to chloroquine. Acta Trop 83:49–61. https://doi.org/10.1016/s0001-706x(02)00056-6
Tönnesmann E, Kandolf R, Lewalter T (2013) Chloroquine cardiomyopathy—a review of the literature. Immunopharmacol Immunotoxicol 35:434–442. https://doi.org/10.3109/08923973.2013.780078
Touret F, de Lamballerie X (2020) Of chloroquine and COVID-19. Antiviral Res 177:104762. https://doi.org/10.1016/j.antiviral.2020.104762
Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, Seidah NG, Nichol ST (2005) Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J 2:69. https://doi.org/10.1186/1743-422X-2-69
Voss A (2020) Official Statement from International Society of Antimicrobial Chemotherapy (ISAC). Available at International Society of Antimicrobial Chemotherapy PublicationWeb https://www.isac.world/news-and-publications/official-isac-statement. Accessed 3 Apr 2020.
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z (2020a) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323:1061–1069. https://doi.org/10.1001/jama.2020.1585
Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G (2020b) Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 30:269–271. https://doi.org/10.1038/s41422-020-0282-0
WHO(World Helth Organization) (2020) Coronavirus disease (COVID-19) Situation Dashboard. Available at World Helth Organization Offical Web https://www.who.int/redirect-pages/page/novel-coronavirus-(covid-19)-situation-dashboard. Accessed 30 Apr 2020
Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y (2020) Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 180:1–11. https://doi.org/10.1001/jamainternmed.2020.0994
Yan Y, Zou Z, Sun Y, Li X, Xu KF, Wei Y, Jin N, Jiang C (2013) Anti-malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Cell Res 23:300–302. https://doi.org/10.1038/cr.2012.165
Yogasundaram H, Putko BN, Tien J, Paterson DI, Cujec B, Ringrose J, Oudit GY (2014) Hydroxychloroquine-induced cardiomyopathy: case report, pathophysiology, diagnosis, and treatment. Can J Cardiol 30:1706–1715. https://doi.org/10.1016/j.cjca.2014.08.016
Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, Akdis CA, Gao YD (2020) Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 75:1730–1741. https://doi.org/10.1111/all.14238
Zou L, Xue Y, Jones M, Heinbockel T, Ying M, Zhan X (2018) The effects of quinine on neurophysiological properties of dopaminergic neurons. Neurotox Res 34:62–73. https://doi.org/10.1007/s12640-017-9855-1
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (Grant Nos. 81822016 and 81771382) to Zhentao Zhang.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zou, L., Dai, L., Zhang, X. et al. Hydroxychloroquine and chloroquine: a potential and controversial treatment for COVID-19. Arch. Pharm. Res. 43, 765–772 (2020). https://doi.org/10.1007/s12272-020-01258-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-020-01258-7