Abstract
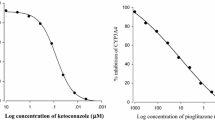
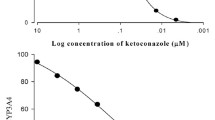
Pioglitazone, a thiazolidinedione antidiabetic drug, inhibits cytochrome P450 (CYP) 2C8 and CYP3A4 enzymes in vitro. This study investigated the effect of pioglitazone on the pharmacokinetics of verapamil and its major metabolite, norverapamil, in rats, after oral administration of verapamil (9 mg/kg) in the presence or absence of pioglitazone (0.3 or 1.0 mg/kg). Pioglitazone altered verapamil pharmacokinetics compared with verapamil alone. The presence of 1.0 mg/kg of pioglitazone significantly (p < 0.05) increased the area under the plasma concentration-time curve (AUC) and the peak concentration (Cmax) of verapamil by 49.0% and 46.8%, respectively, and significantly (p < 0.05) decreased the total plasma clearance (CL/F) of verapamil by 32.8%. The metabolite-parent AUC ratio in the presence of pioglitazone (1.0 mg/kg) significantly (p < 0.05) decreased by 21.9% compared to the control group. Thus, coadministration of pioglitazone inhibited the CYP3A4-mediated metabolism of verapamil.
Similar content being viewed by others
References
Benet, L. Z., Cummins, C. L., and Wu, C. Y., Transporter-enzyme interactions: implications for predicting drug-drug interactions from in vitro data. Curr. Drug. Metab., 4, 393–398 (2003).
Busse, D., Cosme, J., Beaune, P., Kroemer, H. K., and Eichelbaum, M., Cytochromes of the 450 2C subfamily are the major enzymes involved in the O-demethylation of verapamil in humans. Naunyn Schmiedebergs Arch. Pharmacol., 353, 116–121 (1995)
Chilcott, J., Tappenden, P., Jones, M. L., and Wight, J. P., A systematic review of the clinical effectiveness of pioglitazone in the treatment of type 2 diabetes mellitus. Clin. Ther., 23, 1792–1823 (2001).
Cummins, C. L., Jacobsen, W., and Benet, L. Z., Unmasking the dynamic interplay between intestinal P-glycoprotein and CYP3A4. J. Pharmacol. Exp. Ther., 300, 1036–1045 (2002).
Eichelbaum, M., Mikus, G., and Vogelgesang, B., Pharmacokinetics of (+)-, (−)-and (±)-verapamil after intravenous administration. Brit. J. Clin. Pharmacol., 17, 453–458 (1984).
Eichelbaum, M., Remberg, E. G., Schomerus, M., and Dengler, H. J., The metabolism of D, L(14C) verapamil in man. Drug. Metab. Dispos., 7, 145–148 (1979).
Fleckenstein, A., Specific pharmacology of calcium in myocardium, cardiac pacemakers, and vascular smooth muscle. Ann. Rev. Pharmacol. Toxicol., 17, 149–166 (1977).
Gould, B. A,, Mann, S., Kieso, H., Bala Subramanian, V., and Raftery, E. B., The 24 h ambulatory blood pressure profile with verapamil. Circulation, 65, 22–25 (1982).
Kajosaari, L. I., Jaakkola, T., Neuvonen, P. J., and Backman, J. T., Pioglitazone, an in vitro inhibitor of CYP2C8 and CYP3A4, does not increase the plasma concentrations of the CYP2C8 and CYP3A4 substrate repaglinide. Eur. J. Clin. Pharmacol., 62, 217–223 (2006).
Krecic-Shepard, M. E., Barnas, C. R., Slimko, J., and Schwartz, J. B., Faster clearance of sustained release verapamil in men versus women: Continuing observations on sex-specific differences after oral administration of verapamil. Clin. Pharmacol. Ther., 68, 286–292 (2000).
Lewis, G. R., Morley, K. D., Lewis, B. M., and Bones, P. J., The treatment of hypertension with verapamil. NZ. Medical. J., 87, 351–354 (1978).
Prueksaritanont, T., Vega, J. M., Zhao, J., Gagliano, K., Kuznetsova, O., Musser, B., Amin, R. D., Liu, L., Roadcap, A., Dilzer, S., Lasseter, K. C., and Rogers, J. D., Interactions between simvastatin and troglitazone or pioglitazone in healthy subjects. J. Clin. Pharmacol., 41, 573–581 (2001).
Rocci, M. L., and Jusko, W. J., LAGRAN program for area and moments in pharmacokinetic analysis. Computer Programs in Biomedicine, 16, 203–209 (1983).
Schomerus, M., Spiegelhaider, B., Stieren, B., and Eichelbaum, M., Physiologic disposition of verapamil in man. Cardiovasc. Res., 10, 605–612 (1976).
Sahi, J., Black, C. B., Hamilton, G. A., Zheng, X., Jolley, S., Rose, K. A., Gilbert, D., LeCluyse, E. L., and Sinz, M. W., Comparative effects of thiazolidinediones on in vitro P450 enzyme induction and inhibition. Drug Metab. Dispos., 31, 439–446 (2003).
Wacher, V. J., Wu, C. Y., and Benet, L. Z., Overlapping substrate specificities and tissue distribution of cytochrome P450 3A and P-glycoprotein: implications for drug delivery and activity in cancer chemotherapy. Mol. Carcinog., 13, 129–134 (1995).
Walsky, R. L., Gaman, E. A., and Obach, R. S., Examination of 209 drugs for inhibition of cytochrome P450 2C8. J. Clin. Pharmacol., 45, 68–78 (2005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, J.S., Burm, J.P. Effect of pioglitazone on the pharmacokinetics of verapamil and its major metabolite, norverapamil, in rats. Arch. Pharm. Res. 31, 1200–1204 (2008). https://doi.org/10.1007/s12272-001-1289-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-001-1289-z