Abstract
Objective
Recently, a benzofuran derivative for the imaging of β-amyloid plaques, 5-(5-(2-(2-(2-18F-fluoroethoxy)ethoxy)ethoxy)benzofuran-2-yl)- N-methylpyridin-2-amine (18F-FPYBF-2) has been validated as a tracer for amyloid imaging and it was found that 18F-FPYBF-2 PET/CT is a useful and reliable diagnostic tool for the evaluation of AD (Higashi et al. Ann Nucl Med, https://doi.org/10.1007/s12149-018-1236-1, 2018). The aim of this study was to assess the biodistribution and radiation dosimetry of diagnostic dosages of 18F-FPYBF-2 in normal healthy volunteers as a first-in-man study.
Methods
Four normal healthy volunteers (male: 3, female: 1; mean age: 40 ± 17; age range 25–56) were included and underwent 18F-FPYBF-2 PET/CT study for the evaluation of radiation exposure and pharmacokinetics. A 10-min dynamic PET/CT scan of the body (chest and abdomen) was performed at 0–10 min and a 15-min whole-body static scan was performed six times after the injection of 18F-FPYBF-2. After reconstructing PET and CT image data, individual organ time–activity curves were estimated by fitting volume of interest data from the dynamic scan and whole-body scans. The OLINDA/EXM version 2.0 software was used to determine the whole-body effective doses.
Results
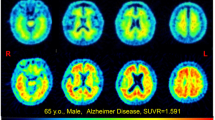
Dynamic PET imaging demonstrated that the hepatobiliary and renal systems were the principal pathways of clearance of 18F-FPYBF-2. High uptake in the liver and the gall bladder, the stomach, and the kidneys were demonstrated, followed by the intestines and the urinary bladder. The ED for the adult dosimetric model was estimated to be 8.48 ± 1.25 µSv/MBq. The higher absorbed doses were estimated for the liver (28.98 ± 12.49 and 36.21 ± 15.64 µGy/MBq), the brain (20.93 ± 4.56 and 23.05 ± 5.03µ Gy/MBq), the osteogenic cells (9.67 ± 1.67 and 10.29 ± 1.70 µGy/MBq), the small intestines (9.12 ± 2.61 and 11.12 ± 3.15 µGy/MBq), and the kidneys (7.81 ± 2.62 and 8.71 ± 2.90 µGy/MBq) for male and female, respectively.
Conclusions
The ED for the adult dosimetric model was similar to those of other agents used for amyloid PET imaging. The diagnostic dosage of 185–370 MBq of 18F-FPYBF-2 was considered to be acceptable for administration in patients as a diagnostic tool for the evaluation of AD.


Similar content being viewed by others
References
Alzheimer Disease International. World Alzheimer Report 2016. 2016. https://www.alz.co.uk/research/WorldAlzheimerReport2016.pdf. Accessed 9 July 2017.
Mathis C, Wang Y, Holt D, Huang G, Debnath M, Klunk W. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J Med Chem. 2003;46:2740–54.
Kudo Y, Okamura N, Furumoto S, Tashiro M, Furukawa K, Maruyama M, et al. 2-(2-[2-Dimethylaminothiazol-5-yl]Ethenyl)-6-(2-[Fluoro]Ethoxy) Benzoxazole: a novel PET agent for in vivo detection of dense amyloid plaques in Alzheimer’s disease patients. J Nucl Med. 2007;48:553 – 61.
Juréus A, Swahn BM, Sandell J, Jeppsson F, Johnson AE, Johnström P, et al. Characterization of AZD4694, a novel fluorinated Abeta plaque neuroimaging PET radioligand. J Neurochem. 2010;114:784 – 94.
Furumoto S, Okamura N, Furukawa K, Tashiro M, Ishikawa Y, Sugi K, et al. A 18F-labeled BF-227 derivative as a potential radioligand for imaging dense amyloid plaques by positron emission tomography. Mol Imaging Biol. 2013;15:497–506.
Rowe CC, Ackerman U, Browne W, Mulligan R, Pike KL, O’Keefe G, et al. Imaging of amyloid beta in Alzheimer’s disease with 18F-BAY94-9172, a novel PET tracer: proof of mechanism. Lancet Neurol. 2008;7:129 – 35.
Choi SR, Golding G, Zhuang Z, Zhang W, Lim N, Hefti F, et al. Preclinical properties of 18F-AV-45: a PET agent for Abeta plaques in the brain. J Nucl Med. 2009;50:1887–94.
Nelissen N, Van Laere K, Thurfjell L, Owenius R, Vandenbulcke M, Koole M, et al. Phase 1 study of the Pittsburgh compound B derivative 18F-flutemetamol in healthy volunteers and patients with probable Alzheimer disease. J Nucl Med. 2009;50:1251–9.
Vandenberghe R, Adamczuk K, Dupont P, Laere KV, Chételat G. Amyloid PET in clinical practice: Its place in the multidimensional space of Alzheimer’s disease. Neuroimage Clin. 2013;2:497–511.
Ono M, Cheng Y, Kimura H, Cui M, Kagawa S, Nishii R, et al. Novel 18F-labeled benzofuran derivatives with improved properties for positron emission tomography (PET) imaging of β-amyloid plaques in Alzheimer’s brains. J Med Chem. 2011;54:2971–9.
Cheng Y, Ono M, Kimura H, Kagawa S, Nishii R, Saji H. A novel 18F-labeled pyridyl benzofuran derivative for imaging of beta-amyloid plaques in Alzheimer’s brains. Bioorg Med Chem Lett. 2010;20:6141–4.
Higashi T, Nishii R, Kagawa S, Kishibe Y, Takahashi M, Okina T, et al. 18F-FPYBF-2, a new F-18 labelled amyloid imaging PET tracer - First-in-man volunteer study and clinical evaluation of patients with dementia. Ann Nucl Med. 2018. https://doi.org/10.1007/s12149-018-1236-1.
Stabin MG, Siegel JA. Physical models and dose factors for use in internal dose assessment. Health Phys. 2003;85:294–310.
Stabin MG, Siegel JA. RADAR Dose Estimate Report: A Compendium of Radiopharmaceutical Dose Estimates Based on OLINDA/EXM Version 2.0. J Nucl Med. 2018;59:154–160.
Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med. 2005;46:1023–7.
ICRP, 2002. Basic anatomical and physiological data for use in radiological protection reference values. ICRP Publication 89. Ann ICRP 32 (3–4).
ICRP, 2007. The 2007 Recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann ICRP 37 (2–4).
Scheinin NM, Tolvanen TK, Wilson IA, Arponen EM, Nagren KA, Rinne JO. Biodistribution and radiation dosimetry of the amyloid imaging agent 11C-PIB in humans. J Nucl Med. 2007;48:128 – 33.
O’Keefe GJ, Saunder TH, Ng S, Ackerman U, Tochon-Danguy HJ, Chan JG, et al. Radiation dosimetry of beta-amyloid tracers 11C-PiB and 18F-BAY94-9172. J Nucl Med. 2009;50:309 – 15.
Lin KJ, Hsu WC, Hsiao IT, Wey SP, Jin LW, Skovronsky D, et al. Whole-body biodistribution and brain PET imaging with [18F]AV-45, a novel amyloid imaging agent—a pilot study. Nucl Med Biol. 2010;37:497–508.
Koole M, Lewis DM, Buckley C, Nelissen N, Vandenbulcke M, Brooks DJ, et al. Whole-body biodistribution and radiation dosimetry of 18F-GE067: a radioligand for in vivo brain amyloid imaging. J Nucl Med. 2009;50:818 – 22.
ICRP Radiation dose to patients from radiopharmaceuticals: a compendium of current information related to frequently used substances. 2015. ICRP Publication 128. Ann. ICRP 44(2S).
Kase K. Radiation protection principles of NCRP. Health Phys. 2004;87:251–7.
Macey D, Williams L, Breitz H, Liu A, Johnson T, Zanzonico P. A primer for radioimmunotherapy and radionuclide therapy. AAPM Rep. 2001;71. https://www.aapm.org/pubs/reports/rpt_71.pdf.
Acknowledgements
This work was supported by the Japan Society for the Promotion of Science (JSPS) through the “Funding Program for Next Generation World-Leading Researchers (NEXT Program, LS060),” initiated by the Council for Science and Technology Policy (CSTP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflicts of interest were disclosed with regard to this study.
Additional information
This study is registered in UMIN Clinical Trials Registry (UMIN-CTR) as UMIN study ID: UMIN000010304, UMIN000012297, and UMIN000012299.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nishii, R., Higashi, T., Kagawa, S. et al. 18F-FPYBF-2, a new F-18 labelled amyloid imaging PET tracer: biodistribution and radiation dosimetry assessment of first-in-man 18F-FPYBF-2 PET imaging. Ann Nucl Med 32, 256–263 (2018). https://doi.org/10.1007/s12149-018-1240-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-018-1240-5