Abstract
Alzheimer’s disease (AD) is a devastating neurodegenerative disorder affecting the elderly. Current clinical diagnostic tools are often ineffective in accurately diagnosing AD. However, new advances in diagnostic imaging, particularly positron emission tomography (PET) amyloid imaging, have shown increased sensitivity and specificity, as well as high inter-reader agreement. The most commonly studied tracer, PiB-C11, has shown high affinity binding to amyloid, but is limited in its use outside of research due to its short half-life. Instead, development of other PET ligands with increased half-life, such as fluorine-18-labeled (18F) tracers, allows for more widespread use of PET in clinical settings. In particular, recent phase II and III trials of 18F-florbetaben have demonstrated the high accuracy of this PET tracer in identifying amyloid accumulation. This paper will examine the techniques of amyloid imaging, focusing particularly on the recently approved 18F-florbetaben.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder and the most common form of dementia in the elderly [1]. According to the US Centers for Disease Control and Prevention (CDC), AD is the fifth leading cause of death for persons aged 65 years and older [2].
Alzheimer’s disease may occur sporadically or as a result of rare genetic mutations that produce an autosomal dominant form of the disease. Clinical features of AD include amnesic memory impairment, language deterioration and visuospatial deficits, as well as functional and behavioral disturbances [1]. Treatment of AD includes cholinesterase inhibitors such as donepezil, galantamine, and rivastigmine, with mixed evidence of efficacy that vary depending on symptom. For example, one meta-analysis found all three drugs to have similar efficacy in treating cognition, but favored donepezil over galantamine when treating behavior [3].
Early diagnosis is important, with one study reporting that identifying and treating AD patients at an early stage could result in cost savings and health benefits, such a reduction in money spent in services and care for patients and families [4]. The establishment of probable AD or other types of dementia often relies on clinical criteria set forth in the 1980s by the National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) and the Alzheimer’s Disease and Related Disorders Association (ADRDA). Differentiation between AD, other dementias and normal decline is based on medical history, clinical examinations and laboratory studies, as well as genetic, neuropsychological and pathophysiological testing [5]. However, clinical diagnosis remains imperfect, with one meta-study finding high variability of sensitivity and specificity for clinical diagnoses criteria, ranging from 53.0 to 100.0% and 55.0 to 99.0%, respectively. For those studies that included neuropathological verification as the gold standard of diagnosis, clinical criteria NINCDS–ADRDA produced a sensitivity of 76–93% and a specificity of 55–91% [6].
Biomarkers and Diagnostic Methods
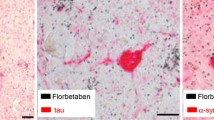
Although the underlying etiology of AD is not fully understood, several potential biomarkers have been identified in the disease progression, such as beta-amyloid and tau proteins. Such biomarkers can be measured either in cerebrospinal fluid (CSF) and, more recently, directly in brain tissue using specialized imaging. Beta-amyloid peptide is currently believed to be an important component of the disease process and the amyloid plaques that result from the accumulation of the protein are considered to be one of the hallmarks of AD. Furthermore, these plaques have become a vital part of neuropathological diagnostic criteria, with current imaging techniques using radiolabeled tracers that bind to the amyloid peptides of amyloid plaques, potentially allowing physicians to directly measure plaque pathology through the use of diagnostic imaging [7]. In fact, the International Working Group (IWG-2) stressed the utility of biomarkers for diagnosis of AD in cases where diagnosis may not have previously been possible. Specifically, they state that tools like magnetic resonance imaging (MRI), positron emission tomography (PET), and CSF analyses have allowed for diagnosis in the “prodromal stage”, before dementia has even appeared [8].
As stated, one method in diagnosing AD is the measurement of biomarkers in CSF. Specifically, AD is associated with an increase in total tau (T-tau) and phosphorylated tau (P-tau), and a decrease in amyloid β-protein 1–42 (Aβ1–42). A study comparing the diagnostic accuracy of MRI, single-photon emission computed tomography (SPECT), fluorodeoxyglucose-positron emission tomography (FDG-PET), and CSF, found higher percentages of positive results using CSF biomarkers (94%), compared with MRI (77.4%) and cerebral blood flow-single-photon emission computed tomography (CBF-SPECT) (81.6%). At Clinical Dementia Rating (CDR) 0.5, 90.0% of patients had positive results using CSF biomarkers; specifically 87.5% with P-tau and 86.7% with T-Tau. Overall, the positive percentage for CSF biomarkers was 95.2% [9]. Another study found CSF biomarkers produced a sensitivity of 89.4% and specificity of 70.5% [10].
In regards to specific biomarkers, a meta-analysis found that T-tau and P-tau produced a sensitivity of 73.3–86.0% and specificity from 70.0 to 92.4%. Combined CSF T-tau and P-tau produced a sensitivity of 81.0% and specificity of 91.0%. CSF Aβ1–42 showed a pooled sensitivity of 85.0 to −100.0% and a specificity of 63.0–90.8%. Finally, combined T-tau, P-tau, and Aβ produced a sensitivity of 85.0–90.0%, with no specificity reported [6].
Functional Imaging
Functional imaging can capture both static and dynamic brain activities in living patients, allowing for measurements of brain physiological processes such as oxygen use, blood perfusion, and glucose metabolism. The most frequently used imaging techniques in identifying and tracking dementia include MRI, SPECT and PET. PET in particular uses positron emitters to label specific brain processes. These positrons are unstable and interact with electrons within brain tissue, producing photons and thus measurable signals [11].
Fluorine-18 (18F)-labeled compounds in general have been shown to be an important group of radiotracers. As AD is associated with reduced glucose metabolism due to reduced cellular activity, 18F fluorodeoxyglucose (FDG) allows for the measurement of changes in glucose metabolism in the brain. This tracer is intravenously injected, phosphorylated in glucose-consuming cells, and then retained in these cells. FDG uptake in a resting state is primarily driven by basal neuronal activity, reflective of neuronal integrity. Thus, AD patients show reduced FDG-PET signal in regions associated with degeneration: specifically in the temporal–parietal, posterior cingulate and frontal cortex—regions associated with memory and orientation [11]. Its long half-life also allows for longer intervals between production and injection. Furthermore, the labeled specific activities of 18F tracers can be increased to levels higher than those of 11C tracers, meaning significantly lower amounts of unlabeled ligand are needed to be injected [7].
Amyloid Imaging
Developments in molecular imaging using PET have also allowed for visualization of fibrillar beta-amyloid plaques in the brains of living AD patients. However, although amyloid PET imaging has allowed for a better understanding of the accumulation of beta-amyloid in vivo, the tracers’ molecular mechanisms and binding properties in both AD and healthy brains is not yet fully understood [12, 13].
The first amyloid-imaging agent to be successfully used in humans was 18fluoro-labeled 1,1-dicyano-2-[6-(dimethylamino)-2-naphthalenyl]propene (FDDNP). This compound was the fluorinated derivative of a cell membrane dye and was able to bind in vitro to beta-amyloid, tau and prion proteins [7].
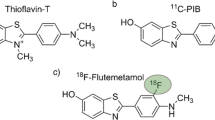
Current amyloid tracers belong to a variety of chemical classes such as thioflavin T (including 11C-PIB, 18F-flutemetamol, and 11C-AZD2184), stilbenes (18F-AV-1, 18F-AV-45, and 11C-SB-13), benzoxazoles (11C-BF-227 and 18F-BF-227), and benzofurans (18F-AZD4694). Researchers theorize that the different chemical classes suggest that these tracers differ in how they bind to fibrillar beta-amyloid, as well as other forms of beta-amyloid [12, 13].
PiB-C11
Currently, the most widely studied tracer is PiB-C11, the first agent to show clear retention in known regions associated with AD amyloid pathology. PiB-C11 binds to both extracellular and intravascular fibrillar amyloid deposits, but not to neurofibrillary tangles (NFTs) or Lewy Bodies. FDDNP, however, does bind to NFTs, thus serving as a potential complement to PiB-C11 amyloid scans. The tracer also binds non-specifically to white matter [7]. However, while cortical retention of the tracer is higher in AD, PiB-C11 shows high variability of binding and no correlation with disease severity [14]. In terms of uptake characteristics, one study reported a PET time of 20, 50-min post-intravenous injection [15].
In the first human study of PiB-C11, researchers found that AD patients showed significantly increased retention in areas of the cortex known to accumulate high levels of amyloid in AD. Specifically, PiB-C11 retention was increased most prominently in frontal cortex, as well as the striatum and parietal, temporal, and occipital cortex. Furthermore, retention was not significantly increased in regions known to develop little amyloid accumulation in the disease process [16].
The primary drawback of PiB-C11 is its 20-min half-life, limiting its broader clinical application [15, 17]. However, 18F-labeled PET tracers have the potential for more clinical use due to their longer half-life of 110 min [15].
18F Tracers
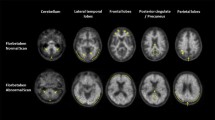
In terms of imaging characteristics, a negative 18F-amyloid scan shows distinctive binding in white matter, while positive scans show binding in cortical gray matter that obscures the typical white matter pattern; in fact, white matter uptake is greater for 18F tracers than for Pib-C11. More specifically, a negative scan will display a clear image of the corpus callosum and pons in a midline sagittal slice, and transverse slices will display normal white matter patterns. A separation of hemispheric activity will also be seen, particularly in the medial orbitofrontal and precuneus areas [14].
Currently, three 18F-labeled amyloid tracers have been evaluated in clinical studies and approved for use by the FDA: 18F-AV-45 (18F-florbetapir; AMYViD™), 18F-flutemetamol (Vizamyl™) and 18F-AV-1 (18F-florbetaben; NeuraCeq™) [12, 13].
Florbetapir
18F-florbetapir ((E)-4-(2-(6-(2-(2-(2-[18F]fluoroethoxy)ethoxy)ethoxy)pyridin-3-yl)vinyl)-N-methylbenzenamine) has been shown to have high affinity, specific binding to amyloid plaques of 3.1 nM [18]. In regards to uptake characteristics, PET scan time has been reported as 10, 50 min after intravenous injection of F18-florbetapir [19].
An early phase human study of 18F-florbetapir found an accumulation of the tracer in cortical regions such as the frontal cortex, temporal cortex, and precuneus, which were expected to be high in beta-amyloid deposition. In contrast, the controls showed tracer accumulation in predominantly white matter area [20].
Another study found cortical retention of 18F-florbetapir was highest in subjects with AD, intermediate in subjects with mild cognitive impairment (MCI), and lowest in cognitively normal subjects, regardless of whether the assessment was via the standardized uptake value ratio (SUVR), visual ratings or binary classification [21].
In one study comparing 18F-florbetapir PET with postmortem histopathology, researchers found high correlations between the autopsy and imaging findings from AD subjects. Specifically, of the fifteen participants that met the pathological criteria for AD at autopsy, fourteen had positive 18F-florbetapir scans. Of the fourteen participants found pathologically negative for AD at autopsy, all fourteen were read as negative on 18F-florbetapir PET [22].
Another study examining correlations with other diagnostic techniques found 18F-florbetapir, similar to PiB-C11, showed high in vivo agreement with postmortem histopathology with sensitivities and specificities exceeding 95%. When comparing 18F-florbetapir to PiB-C11, researchers found similar binding characteristics, with both ligands able to discriminate healthy controls from mild AD, although there was less overlap with PiB-C11. Researchers theorized this may have been due to greater relative non-specific white matter uptake of 18F-florbetapir compared to cortical uptake. In addition, while all brain regions displayed high correlation between the ligands, the SUVR ranges between the lowest and highest values was larger for the PiB-C11 PET scans [15].
Flutemetamol
18F-flutemetamol is a PiB analog developed by GE Healthcare and was developed as an F18 amyloid-imaging agent that would not have the limitations of C11 agents. Sensitivity and specificity were found to be 93.1% and 93.3%, respectively [7]. In terms of imaging characteristics, 18F-flutemetamol shows high intensities in the pons and cerebellar white matter. Furthermore, the center of high-intensity regions show another region of low intensity associated with the fourth ventricle [23]. One study found cerebral cortical uptake of 18F-flutemetamol to be strongly correlated with tissue biopsy beta-amyloid levels. Researchers also found 18F-flutemetamol uptake ratios in PET images to be highly specific for abnormal amyloid deposits in the brain, with moderate to high sensitivity. Single doses were well tolerated. In terms of uptake characteristics, PET imaging was performed 90-min post-injection and completed within 20 min [24].
Florbetaben
18F-florbetaben was the first 18F-labeled Aβ-tracer studied in humans (FBB; trans-4-(N-methyl-amino)-4″(2-(2-(2-[18F] fluoro-ethoxy)ethoxy)-ethoxy)stilbene). In a process described by Zhang et al. [25, 26], radiosynthesis of 18F-florbetaben involved the radiolabeling of a non-radioactive precursor (BOC-Stilbenmesylate) with 18F, followed by acid hydrolysis and semi-preparative high-performance liquid chromatography (HPLC) for purification. Similar to florbetapir, 18F was linked to stilbene through a polyethylene glycol (PEG), as PEG not only lowered the lipophilicity, but also improved overall bioavailability.
The compound has shown specific binding to beta-amyloid plaques in vivo, with a half maximal inhibitory concentration (IC50) of 146 nM for inhibition of [125I]IMPY binding [25, 27]. Another study found the tracer had high affinity binding (K i) to beta-amyloid, with a K i of 6.7 nM, and did not show binding to tangles, Pick Bodies, Lewy Bodies, or glial cytoplasmic inclusions [17]. Early animal studies found that initial brain uptake of 18F-florbetaben was high at 4.77% injected dose (ID)/g 2-min post-injection, and blood serum clearance was rapid at 3.1 l/h/kg. Furthermore, bone uptake was low at 4.64% ID/g at 4-h post-injection. Toxicity trials have found 18F-florbetaben to be well tolerated with no mutagenic properties discovered [25]. In terms of uptake characteristics, PET imaging was completed in 20 min, following a 90- and 110-min post-injection period [28, 29].
Proof of this mechanism was first demonstrated in 2008 through PET imaging of 15 AD patients, five patients with frontotemporal lobar degeneration (FTLD) and 15 healthy controls. Cortical binding produced a robust separation of AD patients from both patients with FTLD and controls, using visual image interpretation or a simple semi-quantitative measure from a 20-min PET scan. Furthermore, researchers found widespread neocortical binding, particularly in the posterior cingulate and frontal cortex, with relative sparing of sensorimotor, occipital and medial temporal cortex.
A phase 0 study of 18F-florbetaben PET found that 9 in 10 AD patients were amyloid positive, with high inter-reader agreement. Neocortical SUVRs were significantly higher in the AD patients’ frontal cortex, lateral temporal cortex, occipital cortex and anterior and posterior cingulate cortices as well as the parietal cortex [30].
A phase II diagnostic study was designed to assess and refine PET scan acquisition and assessment techniques, compare different imaging time points, develop a visual assessment algorithm and develop a quantitative assessment tool. Diagnostic efficacy did not differ significantly across the tested imaging periods, suggesting an added benefit of flexibility in the clinical setting [31]. The clinical phase II study confirmed the initial efficacy findings of the phase 1 studies in a larger study population, including individuals of varying age and different race. Based on these results, a visual assessment methodology and reader training was developed to be applied in future clinical practice. This methodology has been used successfully in the pivotal efficacy studies.
Another study comparing 18F-florbetaben to PiB-C11 found a high correlation between the two tracers in terms of high retention in the cortical and subcortical gray matter and lower coefficients in white matter. Researchers also found that both radiotracers were able to robustly distinguish AD from healthy controls, even with a short PET scan, which would be more easily tolerated by elderly patients. Researchers also noted that while there were no differences in white matter retention for both healthy controls and AD participants using either tracer, the frontal cortex to white matter ratios for PiB-C11 were higher in healthy controls (0.77) and AD (1.45) participants compared to the ratios for 18F-florbetaben in healthy controls and AD (0.70 and 1.12, respectively) [32].
Another study found 18F-florbetaben to be useful in distinguishing AD from healthy controls, as well as other neurodegenerative disorders. Specifically, 96% of patient with AD and 60% of patients with MCI displayed broadly distributed cortical 18F-florbetaben retention, compared to 9% of patients with FTLD, 25% of patients with vascular dementia (VaD), 29% of patients with dementia with Lewy bodies (DLB), and 16% of healthy controls. Sensitivity and specificity was found to be 97% and 88%, respectively [29].
One study found 18F-florbetaben to be effective in assessing brain beta-amyloid levels in individuals with MCI. 45 patients with MCI underwent FBB positron emission tomography. At baseline, 24 (53%) patients with MCI were FBB+. In 2 years, 18 (75%) FBB+ patients progressed to AD compared with 2 (9.5%) FBB-patients, yielding a predictive accuracy of 83% [95% confidence interval (CI) 61–94%] [32]. The florbetaben phase III trial was an open-label, non-randomized study designed to evaluate the efficacy and safety of florbetaben PET imaging for the detection/exclusion of cerebral beta-amyloid. Subjects with a low probability of cerebral beta-amyloid deposition [e.g., non-demented volunteers (NDVs)] and subjects with a high probability of beta-amyloid deposition (e.g., subjects diagnosed with AD or DLB) were included in the trial. Determination of the presence or absence of florbetaben uptake in the PET scan was compared to postmortem histopathology as the standard of truth. Ten young healthy volunteers were included as additional negative controls. The design of the florbetaben phase III study served two different goals: the regional tissue-matched analysis with MRI co-registration was designed as a “target validation” study, aiming to provide pivotal support for the validity of florbetaben PET imaging to detect amyloid aggregates in precisely the same tissue as that examined by histopathology. The subject-level analysis of the florbetaben PET images without MRI co-registration was designed to provide pivotal support for the visual assessment methodology intended for clinical use. In areas known to show beta-amyloid plaques more frequently in AD (i.e., frontal cortex, anterior cingulate and posterior cingulate/precuneus area), the regional tissue-matched analysis showed a sensitivity of 82–90% and specificity of 86–95%. Sensitivity was lower (57%) but specificity higher (100%) for the hippocampus, resulting in an overall sensitivity of 77.4% (95% CI 65.3–89.4%) and a specificity of 94.2% (95% CI 88.6–99.8%) for all regions of interest. In the regional tissue-matched quantitative florbetaben PET assessment, significantly higher SUVRs were found for regions confirmed to have histopathological evidence of beta-amyloid compared with regions that were scored negatively for beta-amyloid, with the exception of the hippocampus/parahippocampal gyrus, consistent with the visual assessment suggesting that the unique anatomy of this region limits the reliability of PET assessment. Furthermore, PET scan reads on a subject-level (whole brain) were investigated to determine the sensitivity and specificity of florbetaben in this setting. The results from the visual assessment using the method applicable for clinical routine were compared with the neuropathological assessment of absence/presence of beta-amyloid plaques according to Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) criteria. A sensitivity of 100% (95% CI 80.5–100.0%), a specificity of 91.67% (95% CI 80.6–100.0%), and an almost perfect across-reader agreement [estimate interrater reliability (κ) = 0.870] was obtained for the first 31 deceased subjects and the image analyses from 10 young healthy volunteers who were considered to be negative for beta-amyloid. (The results from the interim report were subsequently confirmed in a larger cohort of subjects and were applied for approvals in the EU and US. Moreover, the addition of young, healthy volunteers was no longer needed as a sufficiently high number of amyloid-negative subjects became available for the analyses).
Conclusions
Recent guidelines have supported the use of amyloid imaging in a specific set of clinical situations, including patients with persistent or progressive MCI, patients who meet the criteria for possible AD, and patients with progressive dementia and atypical early onset dementia. However, some authors have stated that amyloid imaging is inappropriate in cases including: patients with probable AD with typical age of onset; patients with a positive family history or apolipoprotein 4 (APOE4) mutation; patients with unconfirmed cognitive complaints, in lieu of genotyping, or as a means of determining severity. They also could not support the utility of amyloid imaging for non-medical purposes such as assessing competency in a legal context or assessing ability to perform activities for daily living. These guidelines noted the clinical limitations of C11 due to its short half-life, stating that 18F-labeled PET half-life allows for better incorporation into routine practice. Furthermore, authors have argued that while the clinical utility based on change in case management or change in diagnosis has not yet been established, amyloid PET imaging may be useful in excluding AD in cases of MCI complicated with vascular, traumatic or medical causes [18].
18F-florbetaben is a promising approved PET radiotracer allowing for more widespread use of amyloid imaging in clinical settings. The tracer has the potential for early, reliable detection of AD disease and can aid in facilitating specific treatment decisions, due to its improved high sensitivity and specificity over previous clinical criteria.
References
Cummings JL. Alzheimer’s disease. N Engl J Med. 2004;351:56–67.
Miniño AM. Death in the United States, 2011. NCHS Data Brief, 115. http://www.cdc.gov/nchs/data/databriefs/db115.htm. Accessed Nov 11, 2014.
Hansen RA, Gartlehner G, Webb AP, Morgan LC, Moore CG, Jonas DE. Efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer’s disease: a systematic review and meta-analysis. Clin Interv Aging. 2008;3:211–25.
Banarjee S, Wittenberg R. Clinical and cost effectiveness of services for early diagnosis and intervention in dementia. Int J Geriatr Psychiatry. 2009;24:748–54.
McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–9.
Gaugler JE, Kane RL, Johnston JA, Sarsour K. Sensitivity and specificity of diagnostic accuracy in Alzheimer’s disease: a synthesis of existing evidence. Am J Alzheimers Dis Other Demen. 2013;28:337–47.
Nair AK, Sabbagh MN. Amyloid imaging. In: Nair AK, Sabbagh MN, editors. Geriatric neurology. Chichester: Wiley-Blackwell; 2014.
Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–29 (Erratum: Lancet Neurol. 2014;13:757).
Morinaga A, Ono K, Ikeda T, et al. A comparison of the diagnostic sensitivity of MRI, CBF-SPECT, FDG-PET and cerebrospinal fluid biomarkers for detecting Alzheimer’s Disease in a memory clinic. Dement Geriatr Cogn Disord. 2010;30:285–92.
Cure S, Abrams K, Belger M, Dell’agnello G, Happich M. Systematic literature review and meta-analysis of diagnostic test accuracy in Alzheimer’s Disease and other dementia using autopsy as standard of truth. J Alzheimers Dis. 2014;42:169–82.
Fleisher AS, Drzezga A. Functional Imaging in Dementia. In: Nair AK, Sabbagh MN, editors. Geriatric neurology. Chichester: Wiley-Blackwell; 2014.
Mason NS, Mathis CA, Klunk WE. Positron emission tomography radioligands for in vivo imaging of Aβ plaques. J Labelled Comp Radiopharm. 2013;56:89–95.
Rowe CC, Villemagne VL. Brain amyloid imaging. J Nucl Med. 2011;52:1733–40.
Wolk DA, Zhang Z, Boudhar S, Clark CM, Pontecorvo MJ, Arnold SE. Amyloid imaging in Alzheimer’s disease: comparison of florbetapir and Pittsburgh compound-B positron emission tomography. J Neurol Neurosurg Psychiatry. 2012;83:923–6.
Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–19.
Rowe CC, Ackerman U, Browne W, et al. Imaging of amyloid beta in Alzheimer’s disease with 18F-BAY94-9172, a novel PET tracer: proof of mechanism. Lancet Neurol. 2008;7:129–35.
Johnson KA, Sperling RA, Gidicsin CM, et al. Florbetapir (F18-AV-45) PET to assess amyloid burden in Alzheimer’s disease dementia, mild cognitive impairment, and normal aging. Alzheimers Dement. 2013;9:S72–83.
Siderowf A, Pontecorvo MJ, Shill HA, et al. PET imaging of amyloid with Florbetapir F18 and PET imaging of dopamine degeneration with 18F-AV-133 (florbenazine) in patients with Alzheimer’s disease and Lewy body disorders. BMC Neurol. 2014;14:79.
Wong DF, Rosenberg PB, Zhou Y, et al. In vivo imaging of amyloid deposition in Alzheimer disease using the radioligand 18F-AV-45 (florbetapir [corrected] F 18). J Nucl Med. 2010;51:913–20.
Johnson KA, Minoshima S, Bohnen NI, Alzheimer’s Association; Society of Nuclear Medicine and Molecular Imaging; Amyloid Imaging Taskforce, et al. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. Alzheimers Dement. 2013;9:e-1–16.
Clark CM, Schneider JA, Bedell BJ, AV45-A07 Study Group, et al. Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA. 2011;305:275–83.
Lundqvist R, Lilja J, Thomas BA, et al. Implementation and validation of an adaptive template registration method for 18F-flutemetamol imaging data. J Nucl Med. 2013;54:1472–8.
Leinonen V, Rinne JO, Virtanen KA, et al. Positron emission tomography with [18F]flutemetamol and [11C]PiB for in vivo detection of cerebral cortical amyloid in normal pressure hydrocephalus patients. Eur J Neurol. 2013;20:1043–52.
Barthel H, Sabri O. Florbetaben to trace amyloid-β in the Alzheimer brain by means of PET. J Alzheimers Dis. 2011;26:117–21.
Zhang W, Oya S, Kung MP, Hou C, Maier DL, Kung HF. F-18 Polyethyleneglycol stilbenes as PET imaging agents targeting Abeta aggregates in the brain. Nucl Med Biol. 2005;32:799–809.
Ni R, Gillberg PG, Bergfors A, Marutle A, Nordberg A. Amyloid tracers detect multiple binding sites in Alzheimer’s disease brain tissue. Brain. 2013;136:2217–27.
Ong K, Villemagne VL, Bahar-Fuchs A, et al. (18)F-florbetaben Aβ imaging in mild cognitive impairment. Alzheimers Res Ther. 2013;5:4.
Villemagne VL, Ong K, Mulligan RS, et al. Amyloid imaging with 18F-florbetaben in Alzheimer disease and other dementias. J Nucl Med. 2011;52:1210–7.
Barthel H, Luthardt J, Becker G, et al. Individualized quantification of brain β-amyloid burden: results of a proof of mechanism phase 0 florbetaben PET trial in patients with Alzheimer’s disease and healthy controls. Eur J Nucl Med Mol Imaging. 2011;38:1702–14.
Barthel H, Gertz HJ, Dresel S, et al. Florbetaben Study Group. (18F) in patients with Alzheimer’s disease and healthy controls: a multicentre phase 2 diagnostic study. Lancet Neurol. 2011;10:424–35.
Villemagne VL, Mulligan RS, Pejoska S, et al. Comparison of 11C-PiB and 18F-florbetaben for Aβ imaging in ageing and Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2012;39:983–9.
Ong KT, Villemagne VL, Bahar-Fuchs A, et al. Aβ imaging with 18F-florbetaben in prodromal Alzheimer’s disease: a prospective outcome study. J Neurol Neurosurg Psychiatry. 2014. doi:10.1136/jnnp-2014-308094
Acknowledgments
No funding or sponsorship was received for this study or publication of this article. This research is supported by the Banner Sun Health Research Institute and National Institute on Aging (P30 AG19610). During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from comments received were made by the author based on their scientific and editorial merit.
Conflict of interest
M. N. Sabbagh is an investigator for Piramal Imaging Limited, Mumbai, India (florbetaben F18); GE Healthcare, Princeton, NJ, USA (flutametamol), Avid Radiopharmaceuticals, Philadelphia, PA, USA (florbetapir); Lilly, Indianapolis, IN, USA Functional Neuromodulation Ltd, Minneapolis, USA; Merck, Whitehouse Station, NJ, USA; F. Hoffmann-La Roche Ltd, Basel, Switzerland; Genentech, Inc., San Francisco, CA, USA; Navidea Biopharmaceuticals, Dublin, OH, USA; Neuronix, Yoqnea’m, Israel; and Takeda Pharmaceutical Company Limited, Osaka, Japan. M. N. Sabbagh also serves as an advisor to Piramal Imaging Limited, Mumbai India (florbetaben F18); Lilly, Indianapolis IN; Muses Labs, Raleigh, NC, USA; and Biogen Idec, Cambridge, MA, USA. D. Richards declares no conflict of interest.
Compliance with ethics guidelines
The analysis in this article is based on previously conducted studies, and does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Richards, D., Sabbagh, M.N. Florbetaben for PET Imaging of Beta-Amyloid Plaques in the Brain. Neurol Ther 3, 79–88 (2014). https://doi.org/10.1007/s40120-014-0022-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-014-0022-9