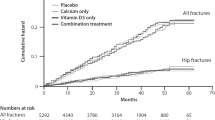
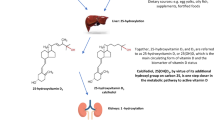
Abstract
Several compounds are produced along the complex pathways of vitamin D3 metabolism, and synthetic analogs have been generated to improve kinetics and/or vitamin D receptor activation. These metabolites display different chemical properties with respect to the parental or native vitamin D3, i.e., cholecalciferol, which has been, so far, the supplement most employed in the treatment of vitamin D inadequacy. Hydrophilic properties of vitamin D3 derivatives facilitate their intestinal absorption and their manageability in the case of intoxication because of the shorter half-life. Calcidiol is a more hydrophilic compound than parental vitamin D3. Active vitamin D analogs, capable of binding the vitamin D receptor evoking vitamin D-related biological effects, are mandatorily employed in hypoparathyroidism and kidney failure with impaired 1α-hydroxylation. They have been shown to increase BMD, supposedly ameliorating calcium absorption and/or directly affecting bone cells, although their use in these conditions is jeopardized by the development of hypercalciuria and mild hypercalcemia. Further studies are needed to assess their overall safety and effectiveness in the long-term and new intermittent regimens, especially when combined with the most effective antifracture agents.

Similar content being viewed by others
References
G. Jones, D.E. Prosser, M. Kaufmann, Cytochrome P450-mediated metabolism of vitamin D. J. Lipid Res. 55, 13 (2014)
M. Imawari, Y. Akanuma, H. Itakura, Y. Muto, K. Kosaka, D.S. Goodman, The effects of diseases of the liver on serum 25-hydroxyvitamin D and on the serum binding protein for vitamin D and its metabolites. J. Lab. Clin. Med. 93, 171 (1979)
J.E. Zerwekh, Blood biomarkers of vitamin D status. Am. J. Clin. Nutr. 87, 1087S–1091S (2008)
L. Cianferotti, C. Marcocci, Subclinical vitamin D deficiency. Best Pract. Res. Clin. Endocrinol. Metab. 26, 523 (2012)
N. Binkley, D. Wiebe, Clinical controversies in vitamin D: 25(OH)D measurement, target concentration, and supplementation. J. Clin. Densitom. 16, 402 (2013)
M. Abboud, D.A. Puglisi, B.N. Davies, M. Rybchyn, N.P. Whitehead, K.E. Brock, L. Cole, C. Gordon-Thomson, D.R. Fraser, R.S. Mason, Evidence for a specific uptake and retention mechanism for 25-hydroxyvitamin D (25OHD) in skeletal muscle cells. Endocrinology 154, 3022 (2013)
F. Molnár, R. Sigüeiro, Y. Sato, C. Araujo, I. Schuster, P. Antony, J. Peluso, C. Muller, A. Mouriño, D. Moras, N. Rochel, 1α,25(OH)2-3-epi-vitamin D3, a natural physiological metabolite of vitamin D3: its synthesis, biological activity and crystal structure with its receptor. Plos One 6, e18124 (2011)
M.F. Holick, Vitamin D deficiency. N. Engl. J. Med. 357, 266 (2007)
C.M. Girgis, R.J. Clifton-Bligh, M.W. Hamrick, M.F. Holick, J.E. Gunton, The roles of vitamin D in skeletal muscle: form, function, and metabolism. Endocr. Rev. 34, 33 (2013)
P. Gerdhem, K.A. Ringsberg, K.J. Obrant, K. Akesson, Association between 25-hydroxy vitamin D levels, physical activity, muscle strength and fractures in the prospective population-based OPRA Study of Elderly Women. Osteoporos. Int. 16, 1425 (2005)
J.K. Lai, R.M. Lucas, M.S. Clements, A.W. Roddam, E. Banks, Hip fracture risk in relation to vitamin D supplementation and serum 25-hydroxyvitamin D levels: a systematic review and meta-analysis of randomised controlled trials and observational studies. BMC Public Health. 10, 331 (2010)
P. Pludowski, M.F. Holick, S. Pilz, C.L. Wagner, B.W. Hollis, W.B. Grant, Y. Shoenfeld, E. Lerchbaum, D.J. Llewellyn, K. Kienreich, M. Soni, Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmun. Rev. 12, 976 (2013)
H.A. Bischoff-Ferrari, E. Giovannucci, W.C. Willett, T. Dietrich, B. Dawson-Hughes, Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am. J. Clin. Nutr. 84, 18 (2006)
M. Wacker, M.F. Holick, Vitamin D—effects on skeletal and extraskeletal health and the need for supplementation. Nutrients. 5, 111 (2013)
B.W. Hollis, C.L. Wagner, Clinical review: the role of the parent compound vitamin D with respect to metabolism and function: Why clinical dose intervals can affect clinical outcomes. J. Clin. Endocrinol. Metab. 98, 4619 (2013)
R.F. Chun, B.E. Peercy, E.S. Orwoll, C.M. Nielson, J.S. Adams, M. Hewison, Vitamin D and DBP: the free hormone hypothesis revisited. J Steroid Biochem Mol Biol. 144, 132 (2014)
J.C. Gallagher, A. Sai, T. Templin 2nd, L. Smith, Dose response to vitamin D supplementation in postmenopausal women: a randomized trial. Ann. Intern. Med. 156, 425 (2012)
B. Dawson-Hughes, A. Mithal, J.P. Bonjour, S. Boonen, P. Burckhardt, G.E. Fuleihan, R.G. Josse, P. Lips, J. Morales-Torres, N. Yoshimura, IOF position statement: vitamin D recommendations for older adults. Osteoporos. Int. 21, 1151 (2010)
M.F. Holick, N.C. Binkley, H.A. Bischoff-Ferrari, C.M. Gordon, D.A. Hanley, R.P. Heaney, M.H. Murad, C.M. Weaver, Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J. Clin. Endocrinol. Metab. 97, 1153 (2012)
R. Rizzoli, S. Boonen, M.L. Brandi, O. Bruyère, C. Cooper, J.A. Kanis, J.M. Kaufman, J.D. Ringe, G. Weryha, J.Y. Reginster, Vitamin D supplementation in elderly or postmenopausal women: a 2013 update of the 2008 recommendations from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Curr. Med. Res. Opin. 29, 305 (2013)
H.A. Bischoff-Ferrari, W.C. Willett, E.J. Orav, P. Lips, P.J. Meunier, R.A. Lyons, L. Flicker, J. Wark, R.D. Jackson, J.A. Cauley, H.E. Meyer, M. Pfeifer, K.M. Sanders, H.B. Stähelin, R. Theiler, B. Dawson-Hughes, A pooled analysis of vitamin D dose requirements for fracture prevention. N. Engl. J. Med. 367, 40 (2012)
K.M. Sanders, A.L. Stuart, E.J. Williamson, J.A. Simpson, M.A. Kotowicz, D. Young, G.C. Nicholson, Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA 303, 1815 (2010)
M. Rossini, S. Adami, O. Viapiana, E. Fracassi, L. Idolazzi, M.R. Povino, D. Gatti, Dose-dependent short-term effects of single high doses of oral vitamin D(3) on bone turnover markers. Calcif. Tissue Int. 91, 365 (2012)
M.R. Haussler, P.W. Jurutka, M. Mizwicki, A.W. Norman, Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)2vitamin D3: genomic and non-genomic mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 25, 543 (2011)
H.F. Deluca, History of the discovery of vitamin D and its active metabolites. Bonekey Rep. 3, 479 (2014)
J. Lund, H.F. DeLuca, Biologically active metabolite of vitamin D3 from bone, liver, and blood serum. J. Lipid Res. 7, 739 (1966)
L.V. Avioli, S.W. Lee, J.E. McDonald, J. Lund, H.F. DeLuca, Metabolism of vitamin D3-3H in human subjects: distribution in blood, bile, feces, and urine. J. Clin. Invest. 46, 983 (1967)
J.W. Blunt, H.F. DeLuca, H.K. Schnoes, 25-hydroxycholecalciferol. A biologically active metabolite of vitamin D3. Biochemistry 7, 3317 (1968)
J.W. Blunt, Y. Tanaka, H.F. DeLuca, Biological activity of 25-hydroxycholecalciferol, a metabolite of vitamin D3. Proc. Natl. Acad. Sci. USA 61, 1503 (1968)
J.W. Blunt, H.F. DeLuca, The synthesis of 25-hydroxycholecalciferol. A biologically active metabolite of vitamin D3. Biochemistry 8, 671 (1969)
G. Ponchon, A.L. Kennan, H.F. DeLuca, “Activation” of vitamin D by the liver. J. Clin. Invest. 48, 2032 (1969)
D.R. Fraser, E. Kodicek, Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature 228, 764 (1970)
D.E. Lawson, D.R. Fraser, E. Kodicek, H.R. Morris, D.H. Williams, Identification of 1,25-dihydroxycholecalciferol, a new kidney hormone controlling calcium metabolism. Nature 230, 228 (1971)
I.T. Boyle, L. Miravet, R.W. Gray, M.F. Holick, H.F. Deluca, The response of intestinal calcium transport to 25-hydroxy and 1,25-dihydroxy vitamin D in nephrectomized rats. Endocrinology 90, 605 (1972)
M.F. Holick, M. Garabedian, H.F. DeLuca, 1,25-dihydroxycholecalciferol: metabolite of vitamin D3 active on bone in anephric rats. Science 176, 1146 (1972)
J.G. Haddad, K.J. Chyu, Competitive protein-binding radioassay for 25-hydroxycholecalciferol. J. Clin. Endocrinol. Metab. 33, 992 (1971)
R. Belsey, H.F. Deluca, J.T. Potts Jr, Competitive binding assay for vitamin D and 25-OH vitamin D. J. Clin. Endocrinol. Metab. 33, 554 (1971)
M.F. Holick, H.K. Schnoes, H.F. DeLuca, Identification of 1,25-dihydroxycholecalciferol, a form of vitamin D3 metabolically active in the intestine. Proc. Natl. Acad. Sci. USA 68, 803 (1971)
A.W. Norman, J.F. Myrtle, R.J. Midgett, H.G. Nowicki, V. Williams, G. Popják, 1,25-dihydroxycholecalciferol: identification of the proposed active form of vitamin D3 in the intestine. Science 173, 51 (1971)
Y. Tanaka, H.F. DeLuca, J. Omdahl, M.F. Holick, Mechanism of action of 1,25-dihydroxycholecalciferol on intestinal calcium transport. Proc. Natl. Acad. Sci USA 68, 1286 (1971)
C.A. Frolik, H.F. Deluca, 1,25-dihydroxycholecalciferol: the metabolite of vitamin D responsible for increased intestinal calcium transport. Arch. Biochem. Biophys. 147, 143 (1971)
M. Garabedian, M.F. Holick, H.F. Deluca, I.T. Boyle, Control of 25-hydroxycholecalciferol metabolism by parathyroid glands. Proc. Natl. Acad. Sci. USA 69, 1673 (1972)
E.B. Mawer, C.M. Taylor, J. Backhouse, G.A. Lumb, S.W. Stanbury, Failure of formation of 1,25-dihydroxycholecalciferol in chronic renal insufficiency. Lancet 1, 626 (1973)
B.S. Chertow, D.J. Baylink, J.E. Wergedal, M.H. Su, A.W. Norman, Decrease in serum immunoreactive parathyroid hormone in rats and in parathyroid hormone secretion in vitro by 1,25-dihydroxycholecalciferol. J. Clin. Invest. 56, 668 (1975)
C.A. Frolik, H.F. DeLuca, The stimulation of 1,25-dihydroxycholecalciferol metabolism in vitamin D-deficient rats by 1,25-dihydroxycholecalciferol treatment. J. Clin. Invest. 52, 543 (1973)
Y. Tanaka, H.F. Deluca, Bone mineral mobilization activity of 1,25-dihydroxycholecalciferol, a metabolite of vitamin D. Arch. Biochem. Biophys. 146, 574 (1971)
L.G. Raisz, C.L. Trummel, M.F. Holick, H.F. DeLuca, 1,25-dihydroxycholecalciferol: a potent stimulator of bone resorption in tissue culture. Science 175, 768 (1972)
M. Garabedian, Y. Tanaka, M.F. Holick, H.F. Deluca, Response of intestinal calcium transport and bone calcium mobilization to 1,25-dihydroxyvitamin D3 in thyroparathyroidectomized rats. Endocrinology 94, 1022 (1974)
R.W. Gray, A.E. Caldas, D.R. Wilz, J. Lemann Jr, G.A. Smith, H.F. DeLuca, Metabolism and excretion of 3H-1,25-(OH)2-vitamin D3 in healthy adults. J. Clin. Endocrinol. Metab. 46, 756 (1978)
T.L. Clemens, G.N. Hendy, R.F. Graham, E.G. Baggiolini, M.R. Uskokovic, J.L. O’Riordan, A radioimmunoassay for 1,25-dihydroxycholecalciferol. Clin. Sci. Mol. Med. 54, 329 (1978)
Consensus Development Conference, Diagnosis, prophylaxis and treatment of osteoporosis. Am. J. Med. 94, 646 (1993)
M.F. Holick, E.J. Semmler, H.K. Schnoes, H.F. DeLuca, 1 -Hydroxy derivative of vitamin D 3: a highly potent analog of 1,25-dihydroxyvitamin D 3. Science 180, 190 (1973)
K. Ueno, Y. Okamiya, T. Makita, S. Kurozumi, H. Kawashima, Y. Hashimoto, Studies on biopharmacological activity of active vitamin D3 analogues (VII) Effect of 1 alpha-hydroxycholecalciferol on renal function in rats and Beagle dogs (Japanese). Nihon. Yakurigaku Zasshi. 75, 617 (1979)
S. Morimoto, S. Imanaka, E. Koh, T. Shiraishi, T. Nabata, S. Kitano, Y. Miyashita, Y. Nishii, T. Ogihara, Comparison of the inhibitions of proliferation of normal and psoriatic fibroblasts by 1 alpha,25-dihydroxyvitamin D3 and synthetic analogues of vitamin D3 with an oxygen atom in their side chain. Biochem. Int. 19, 1143 (1989)
H. Tsurukami, T. Nakamura, K. Suzuki, K. Sato, Y. Higuchi, Y. Nishii, A novel synthetic vitamin D analogue, 2 beta-(3-hydroxypropoxy)1 alpha, 25-dihydroxyvitamin D3 (ED-71), increases bone mass by stimulating the bone formation in normal and ovariectomized rats. Calcif. Tissue Int. 54, 142 (1994)
Y. Tanaka, T. Nakamura, S. Nishida, K. Suzuki, S. Takeda, K. Sato, Y. Nishii, Effects of a synthetic vitamin D analog, ED-71, on bone dynamics and strength in cancellous and cortical bone in prednisolone-treated rats. J. Bone Miner. Res. 11, 325 (1996)
Y. Uchiyama, Y. Higuchi, S. Takeda, T. Masaki, A. Shira-Ishi, K. Sato, N. Kubodera, K. Ikeda, E. Ogata, ED-71, a vitamin D analog, is a more potent inhibitor of bone resorption than alfacalcidol in an estrogen-deficient rat model of osteoporosis. Bone 30, 582 (2002)
P.H. de Freitas, T. Hasegawa, S. Takeda, M. Sasaki, C. Tabata, K. Oda, M. Li, H. Saito, N. Amizuka, Eldecalcitol, a second-generation vitamin D analog, drives bone minimodeling and reduces osteoclastic number in trabecular bone of ovariectomized rats. Bone 49, 335 (2011)
S. Harada, T. Mizoguchi, Y. Kobayashi, Y. Nakamichi, S. Takeda, S. Sakai, F. Takahashi, H. Saito, H. Yasuda, N. Udagawa, T. Suda, N. Takahashi, Daily administration of eldecalcitol (ED-71), an active vitamin D analog, increases bone mineral density by suppressing RANKL expression in mouse trabecular bone. J. Bone Miner. Res. 27, 461 (2012)
K.H. Lau, D.J. Baylink, Vitamin D therapy of osteoporosis: plain vitamin D therapy versus active vitamin D analog (D-hormone) therapy. Calcif. Tissue Int. 65, 295 (1999)
S. O’Donnell, D. Moher, K. Thomas, D.A. Hanley, A. Cranney, Systematic review of the benefits and harms of calcitriol and alfacalcidol for fractures and falls. J. Bone Miner. Metab. 26, 531 (2008)
M.L. Brandi, S. Minisola, Calcidiol [25(OH)D3]: from diagnostic marker to therapeutical agent. Curr. Med. Res. Opin. 29, 1565 (2013)
L.J. Peppone, S. Hebl, J.Q. Purnell, M.E. Reid, R.N. Rosier, K.M. Mustian, O.G. Palesh, A.J. Huston, M.N. Ling, G.R. Morrow, The efficacy of calcitriol therapy in the management of bone loss and fractures: a qualitative review. Osteoporos. Int. 21, 1133 (2010)
J.A. Eisman, R. Bouillon, Vitamin D: direct effects of vitamin D metabolites on bone: lessons from genetically modified mice. Bonekey Rep. 3, 499 (2014)
L. Ovesen, C. Brot, J. Jakobsen, Food contents and biological activity of 25-hydroxyvitamin D: a vitamin D metabolite to be reckoned with? Ann. Nutr. Metab. 47, 107 (2003)
J.E. Smith, D.S. Goodman, The turnover and transport of vitamin D and of a polar metabolite with the properties of 25-hydroxycholecalciferol in human plasma. J. Clin. Invest. 50, 2159 (1971)
T.C. Stamp, J.M. Round, J.G. Haddad, Effect of oral vitamin D, 25-hydroxycholecalciferol (25-HCC) and whole-body ultra-violet irradiation on plasma 25-HCC levels in man. Clin. Sci. 44, 3P (1973)
T.C. Stamp, Intestinal absorption of 25-hydroxycholecalciferol. Lancet 2, 121 (1974)
J.G. Haddad, T.C. Stamp, Circulating 25-hydroxyvitamin D in man. Am. J. Med. 57, 57 (1974)
J.G. Haddad Jr, S. Rojanasathit, Acute administration of 25-hydroxycholecalciferol in man. J. Clin. Endocrinol. Metab. 42, 284 (1976)
K.S. Jones, I. Schoenmakers, L.J. Bluck, S. Ding, A. Prentice, Plasma appearance and disappearance of an oral dose of 25-hydroxyvitamin D2 in healthy adults. Br. J. Nutr. 107, 1128 (2012)
T.C. Stamp, J.G. Haddad, C.A. Twigg, Comparison of oral 25-hydroxycholecalciferol, vitamin D, and ultraviolet light as determinants of circulating 25-hydroxyvitamin D. Lancet 1, 1341 (1977)
B.W. Hollis, H.R. Conrad, J.W. Hibbs, Changes in plasma 25-hydroxycholecalciferol and selected blood parameters after injection of massive doses of cholecalciferol or 25-hydroxycholecalciferol in non-lactating dairy cows. J. Nutr. 107, 606 (1977)
M.J. Barger-Lux, R.P. Heaney, S. Dowell, T.C. Chen, M.F. Holick, Vitamin D and its major metabolites: serum levels after graded oral dosing in healthy men. Osteoporos. Int. 8, 222 (1998)
A. Jetter, A. Egli, B. Dawson-Hughes, H.B. Staehelin, E. Stoecklin, R. Goessl, J. Henschkowski, H.A. Bischoff-Ferrari, Pharmacokinetics of oral vitamin D(3) and calcifediol. Bone 59, 14 (2014)
K.D. Cashman, K.M. Seamans, A.J. Lucey, E. Stöcklin, P. Weber, M. Kiely, T.R. Hill, Relative effectiveness of oral 25-hydroxyvitamin D3 and vitamin D3 in raising wintertime serum 25-hydroxyvitamin D in older adults. Am. J. Clin. Nutr. 95, 1350 (2012)
S.H. Chou, T.K. Chung, B. Yu, Effects of supplemental 25-hydroxycholecalciferol on growth performance, small intestinal morphology, and immune response of broiler chickens. Poult. Sci. 88, 2333 (2009)
C. Lauridsen, U. Halekoh, T. Larsen, S.K. Jensen, Reproductive performance and bone status markers of gilts and lactating sows supplemented with two different forms of vitamin D. J. Anim. Sci. 88, 202 (2010)
N. Sahin, T.A. Balci, O. Kucuk, M.O. Smith, K. Sahin, Effects of 25-hydroxycholecalciferol and soy isoflavones supplementation on bone mineralisation of quail. Br. Poult. Sci. 50, 709 (2009)
E.A. Hines, J.D. Coffey, C.W. Starkey, T.K. Chung, J.D. Starkey, Improvement of maternal vitamin D status with 25-hydroxycholecalciferol positively impacts porcine fetal skeletal muscle development and myoblast activity. J. Anim. Sci. 91, 4116 (2013)
T.J. Hahn, L.R. Halstead, S.L. Teitelbaum, B.H. Hahn, Altered mineral metabolism in glucocorticoid-induced osteopenia. Effect of 25-hydroxyvitamin D administration. J. Clin. Invest. 64, 655 (1979)
R.M. Francis, M. Peacock, J.H. Storer, A.E. Davies, W.B. Brown, B.E. Nordin, Calcium malabsorption in the elderly: the effect of treatment with oral 25-hydroxyvitamin D3. Eur. J. Clin. Invest. 13, 391 (1983)
S. Russo, L. Carlucci, C. Cipriani, A. Ragno, S. Piemonte, R.D. Fiacco, J. Pepe, V. Fassino, S. Arima, E. Romagnoli, S. Minisola, Metabolic changes following 500 μg monthly administration of calcidiol: a study in normal females. Calcif. Tissue Int. 89, 252 (2011)
M. Peacock, G. Liu, M. Carey, R. McClintock, W. Ambrosius, S. Hui, C.C. Johnston, Effect of calcium or 25OH vitamin D3 dietary supplementation on bone loss at the hip in men and women over the age of 60. J. Clin. Endocrinol. Metab. 85, 3011 (2000)
M. Sosa, P. Láinez, A. Arbelo, M.C. Navarro, The effect of 25-dihydroxyvitamin D on the bone mineral metabolism of elderly women with hip fracture. Rheumatology (Oxford) 39, 1263 (2000)
H.A. Bischoff-Ferrari, B. Dawson-Hughes, E. Stöcklin, E. Sidelnikov, W.C. Willett, J.O. Edel, H.B. Stähelin, S. Wolfram, A. Jetter, J. Schwager, J. Henschkowski, A. von Eckardstein, A. Egli, Oral supplementation with 25(OH)D3 versus vitamin D3: effects on 25(OH)D levels, lower extremity function, blood pressure, and markers of innate immunity. J. Bone Miner. Res. 27, 160 (2012)
M.L. Bianchi, C. Colombo, B.M. Assael, A. Dubini, M. Lombardo, S. Quattrucci, S. Bella, M. Collura, B. Messore, V. Raia, F. Poli, R. Bini, C.V. Albanese, V. De Rose, D. Costantini, G. Romano, E. Pustorino, G. Magazzù, S. Bertasi, V. Lucidi, G. Traverso, A. Coruzzo, A.D. Grzejdziak, Treatment of low bone density in young people with cystic fibrosis: a multicentre, prospective, open-label observational study of calcium and calcifediol followed by a randomised placebo-controlled trial of alendronate. Lancet Respir. Med. 1, 377 (2013)
A. Drincic, E. Fuller, R.P. Heaney, L.A. Armas, 25-Hydroxyvitamin D response to graded vitamin D3 supplementation among obese adults. J. Clin. Endocrinol. Metab. 98, 4845 (2013)
S. Petta, C. Cammà, C. Scazzone, C. Tripodo, V. Di Marco, A. Bono, D. Cabibi, G. Licata, R. Porcasi, G. Marchesini, A. Craxí, Low vitamin D serum level is related to severe fibrosis and low responsiveness to interferon-based therapy in genotype 1 chronic hepatitis C. Hepatology 51, 1158 (2010)
L.V. Avioli, S.J. Birge, S.W. Lee, Effects of prednisone on vitamin D metabolism in man. J. Clin. Endocrinol. Metab. 28, 1341 (1968)
M. Carré, O. Ayigbedé, L. Miravet, H. Rasmussen, The effect of Prednisolone upon the metabolism and action of 25-hydroxy-and 1,25-dihydroxyvitamin D3. Proc. Natl. Acad. Sci. USA 71, 2996 (1974)
S.A. Hamed, Influences of bone and mineral metabolism in epilepsy. Expert Opin. Drug Saf. 10, 265 (2011)
Al Mutair, A.N., Nasrat, G.H., Russell, D.W.: mutation of the CYP2R1 vitamin D 25-hydroxylase in a Saudi Arabian family with severe vitamin D deficiency. J. Clin. Endocrinol. Metab. 97, E2022 (2012)
I. Garcia-Delgado, S. Prieto, L. Gil-Fraguas, E. Robles, J.J. Rufilanchas, F. Hawkins, Calcitonin, etidronate, and calcidiol treatment in bone loss after cardiac transplantation. Calcif. Tissue Int. 60, 155 (1997)
M. Talalaj, L. Gradowska, E. Marcinowska-Suchowierska, M. Durlik, Z. Gaciong, M. Lao, Efficiency of preventive treatment of glucocorticoid-induced osteoporosis with 25-hydroxyvitamin D3 and calcium in kidney transplant patients. Transplant. Proc. 28, 3485 (1996)
J.E. Compston, B. Creamer, Plasma levels and intestinal absorption of 25-hydroxyvitamin D in patients with small bowel resection. Gut 18, 171 (1977)
A.J. Batchelor, G. Watson, J.E. Compston, Changes in plasma half-life and clearance of 3H-25-hydroxyvitamin D3 in patients with intestinal malabsorption. Gut 23, 1068 (1982)
M. Davies, E.B. Mawer, E.L. Krawitt, Comparative absorption of vitamin D3 and 25-hydroxyvitamin D3 in intestinal disease. Gut 21, 287 (1980)
J. Michaud, J. Naud, D. Ouimet, C. Demers, J.L. Petit, F.A. Leblond, A. Bonnardeaux, M. Gascon-Barré, V. Pichette, Reduced hepatic synthesis of calcidiol in uremia. J. Am. Soc. Nephrol. 21, 1488 (2010)
G. Jean, J.C. Terrat, T. Vanel, J.M. Hurot, C. Lorriaux, B. Mayor, C. Chazot, Daily oral 25-hydroxycholecalciferol supplementation for vitamin D deficiency in haemodialysis patients: effects on mineral metabolism and bone markers. Nephrol. Dial. Transplant. 23, 3670 (2008)
H. Schmidt-Gayk, C. Grawunder, W. Tschöpe, W. Schmitt, E. Ritz, V. Pietsch, K. Andrassay, R. Bouillon, 25-hydroxy-vitamin-D in nephrotic syndrome. Lancet 2, 105 (1977)
I.H. de Boer, M.C. Sachs, P.A. Cleary, A.N. Hoofnagle, J.M. Lachin, M.E. Molitch, M.W. Steffes, W. Sun, B. Zinman, J.D. Brunzell, Diabetes Control and Complication Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group. Circulating vitamin D metabolites and kidney disease in type 1 diabetes. J. Clin. Endocrinol. Metab. 97, 4780 (2012)
C. Foresta, G. Strapazzon, L. De Toni, L. Perilli, A. Di Mambro, B. Muciaccia, L. Sartori, R. Selice, Bone mineral density and testicular failure: evidence for a role of vitamin D 25-hydroxylase in human testis. J. Clin. Endocrinol. Metab. 96, E646 (2011)
A. Caniggia, R. Nuti, F. Lore, G. Martini, V. Turchetti, G. Righi, Long-term treatment with calcitriol in postmenopausal osteoporosis. Metabolism. 39, 43 (1990)
A. Caniggia, R. Nuti, F. Loré, G. Martini, B. Frediani, S. Giovani, Total body absorptiometry in postmenopausal osteoporosis patients treated with 1 alpha-hydroxylated vitamin D metabolites. Osteoporos. Int. 3, 181 (1993)
M.W. Tilyard, G.F. Spears, J. Thomson, S. Dovey, Treatment of postmenopausal osteoporosis with calcitriol or calcium. N. Engl. J. Med. 326, 357 (1992)
P. Sambrook, N.K. Henderson, A. Keogh, P. MacDonald, A. Glanville, P. Spratt, P. Bergin, P. Ebeling, J. Eisman, Effect of calcitriol on bone loss after cardiac or lung transplantation. J. Bone Miner. Res. 15, 1818 (2000)
J.C. Gallagher, S.E. Fowler, J.R. Detter, S.S. Sherman, Combination treatment with estrogen and calcitriol in the prevention of age-related bone loss. J. Clin. Endocrinol. Metab. 86, 3618 (2001)
J.C. Gallagher, P.B. Rapuri, L.M. Smith, An age-related decrease in creatinine clearance is associated with an increase in number of falls in untreated women but not in women receiving calcitriol treatment. J. Clin. Endocrinol. Metab. 92, 51 (2007)
Y. Rhee, M. Kang, Y. Min, D. Byun, Y. Chung, C. Ahn, K. Baek, J. Mok, D. Kim, D. Kim, H. Kim, Y. Kim, S. Myoung, D. Kim, S.K. Lim, Effects of a combined alendronate and calcitriol agent (Maxmarvil) on bone metabolism in Korean postmenopausal women: a multicenter, double-blind, randomized, placebo-controlled study. Osteoporos. Int. 17, 1801 (2006)
H.W. Suh, H.O. Kim, Y.S. Kim, S. Sunwoo, J.A. Lee, H.R. Lee, B. Kim, D.H. Kim, Y.S. Choi, Y.S. Cheong, K. Yum, Y.J. Yang, B.Y. Yu, C.H. Cho, S.B. Park, D.H. Shin, Korea Post-Marketing Surveillance Research Group. The efficacy and safety of a combined alendronate and calcitriol agent (maxmarvil): a postmarketing surveillance study in korean postmenopausal women with osteoporosis. Korean. J Fam. Med. 33, 346 (2012)
D.L. Trump, D.M. Potter, J. Muindi, A. Brufsky, C.S. Johnson, Phase II trial of high-dose, intermittent calcitriol (1,25 dihydroxyvitamin D3) and dexamethasone in androgen-independent prostate cancer. Cancer 106, 2136 (2006)
H. Orimo, E. Schacht, The D-hormone analog alfacalcidol: the pioneer beyond the horizon of osteoporosis treatment. J. Rheumatol. Suppl. 76, 4 (2005)
R.M. Francis, I.T. Boyle, C. Moniz, A.M. Sutcliffe, B.S. Davis, G.H. Beastall, R.A. Cowan, N. Downes, A comparison of the effects of alfacalcidol treatment and vitamin D2 supplementation on calcium absorption in elderly women with vertebral fractures. Osteoporos. Int. 6, 284 (1996)
R. Nuti, G. Bianchi, M.L. Brandi, R. Caudarella, E. D’Erasmo, C. Fiore, G.C. Isaia, G. Luisetto, M. Muratore, P. Oriente, S. Ortolani, Superiority of alfacalcidol compared to vitamin D plus calcium in lumbar bone mineral density in postmenopausal osteoporosis. Rheumatol. Int. 26, 445 (2006)
J.Y. Reginster, D. Kuntz, W. Verdickt, M. Wouters, L. Guillevin, C.J. Menkès, K. Nielsen, Prophylactic use of alfacalcidol in corticosteroid-induced osteoporosis. Osteoporos. Int. 9, 75 (1999)
J.D. Ringe, A. Dorst, H. Faber, E. Schacht, V.W. Rahlfs, Superiority of alfacalcidol over plain vitamin D in the treatment of glucocorticoid-induced osteoporosis. Rheumatol. Int. 24, 63 (2004)
L. Dukas, H.A. Bischoff, L.S. Lindpaintner, E. Schacht, D. Birkner-Binder, T.N. Damm, B. Thalmann, H.B. Stähelin, Alfacalcidol reduces the number of fallers in a community-dwelling elderly population with a minimum calcium intake of more than 500 mg daily. J. Am. Geriatr. Soc. 52, 230 (2004)
L. Dukas, E. Schacht, Z. Mazor, H.B. Stähelin, Treatment with alfacalcidol in elderly people significantly decreases the high risk of falls associated with a low creatinine clearance of <65 ml/min. Osteoporos. Int. 16, 198 (2005)
J.D. Ringe, P. Farahmand, E. Schacht, Alfacalcidol in men with osteoporosis: a prospective, observational, 2-year trial on 214 patients. Rheumatol. Int. 33, 637 (2013)
J.C. Gallagher, The effects of calcitriol on falls and fractures and physical performance tests. J. Steroid Biochem. Mol. Biol. 89–90, 497 (2004)
J.D. Ringe, P. Farahmand, E. Schacht, A. Rozehnal, Superiority of a combined treatment of Alendronate and Alfacalcidol compared to the combination of Alendronate and plain vitamin D or Alfacalcidol alone in established postmenopausal or male osteoporosis (AAC-Trial). Rheumatol. Int. 27, 425 (2007)
T. Matsumoto, T. Miki, H. Hagino, T. Sugimoto, S. Okamoto, T. Hirota, Y. Tanigawara, Y. Hayashi, M. Fukunaga, M. Shiraki, T. Nakamura, A new active vitamin D, ED-71, increases bone mass in osteoporotic patients under vitamin D supplementation: a randomized, double-blind, placebo-controlled clinical trial. J. Clin. Endocrinol. Metab. 90, 5031 (2005)
T. Matsumoto, N. Kubodera, ED-71, a new active vitamin D3, increases bone mineral density regardless of serum 25(OH)D levels in osteoporotic subjects. J. Steroid Biochem. Mol. Biol. 103, 584 (2007)
T. Matsumoto, M. Ito, Y. Hayashi, T. Hirota, Y. Tanigawara, T. Sone, M. Fukunaga, M. Shiraki, T. Nakamura, A new active vitamin D3 analog, eldecalcitol, prevents the risk of osteoporotic fractures–a randomized, active comparator, double-blind study. Bone 49, 605 (2011)
H. Hagino, T. Takano, M. Fukunaga, M. Shiraki, T. Nakamura, T. Matsumoto, Eldecalcitol reduces the risk of severe vertebral fractures and improves the health-related quality of life in patients with osteoporosis. J. Bone Miner. Metab. 31, 183 (2013)
M.P. Caraceni, G.C. Gandolini, F. Campanini, M.A. Iannetta, S. Ortolani, Vitamin D metabolites in spasmophilia. Biomed. Pharmacother. 44, 479 (1990)
A. Avenell, J.C. Mak, D. O’Connell, Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst. Rev. 4, CD000227 (2014)
Acknowledgments
This paper was derived from a Working Group meeting supported by the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cianferotti, L., Cricelli, C., Kanis, J.A. et al. The clinical use of vitamin D metabolites and their potential developments: a position statement from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the International Osteoporosis Foundation (IOF). Endocrine 50, 12–26 (2015). https://doi.org/10.1007/s12020-015-0606-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-015-0606-x