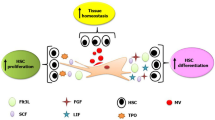
Abstract
The existing approaches of hematopoietic stem cells (HSCs) expansion in vitro were difficult to meet the needs of clinical application. While in vivo, HSCs efficiently self-renew in niche where they interact with three dimension extracellular matrix and stromal cells. Osteoblasts (OBs) are one of most significant stromal cells of HSCs niche. Here, we proposed a three-dimensional environment based on gallic acid grafted-chitosan (2c) scaffold and OBs differentiated from human umbilical cord mesenchymal stem cells (HUMSCs) to recapitulate the main components of HSCs niche. The results of alkaline phosphatase staining and alizarin red staining demonstrated that HUMSCs were successfully induced into OBs. The results showed that the expansions of CD34+cells, CD34+CD38− cells and CD34+CD38−CD45RA−CD49f+CD90+ cells (primitive hematopoietic stem cells, pHSCs) harvested from the biomimetic HSCs niche based on 2c scaffold and OBs (IV) group were larger than those harvested from other three culture groups. Importantly, it was found that the CD34+ cells harvested from IV group had better secondary expansion capability and colony forming potential, indicating better self-renewal ability. In addition, the biomimetic HSCs niche based on 2c scaffold and OBs protected HSCs apoptosis and promoted HSCs division. Taken together, the biomimetic HSCs niche based on 2c scaffold and OBs was an effective strategy for ex vivo expansion of HSCs in clinical scale.
Graphical abstract








Similar content being viewed by others
References
Anthony, B. A., & Link, D. C. (2014). Regulation of hematopoietic stem cells by bone marrow stromal cells. Trends Immunology, 35(1), 32–37.
Arai, F., Hirao, A., & Suda, T. (2006). Regulation of hematopoiesis and its interaction with stem cell niches. International Journal of Hematology, 82(5), 371–376.
Askmyr, M., Sims, N. A., Martin, T. J., & Purton, L. E. (2009). What is the true nature of the osteoblastic hematopoietic stem cell niche? Trends in Endocrinology Metabolism, 20(6), 303–309.
Balise, V. D., Saito-Reis, C. A., & Gillette, J. M. (2020). Tetraspanin scaffold proteins function as key regulators of hematopoietic stem cells. Frontiers in Cell and Developmental Biology, 8(10), 598–607.
Basu, J. (2014). An organ regeneration platform for industrial production of hollow neo-organs. Cells & Biomaterials in Regenerative Medicine, 25(6), 512–520.
Bello, A. B., Park, H., & Lee, S. H. (2018). Current approaches in biomaterial-based hematopoietic stem cell niches. Acta Biomaterialia, 72(4), 1–15.
Bourgine, P. E., Klein, T., Paczulla, A. M., Shimizu, T., Kunz, L., Kokkaliaris, K. D., Coutu, D. L., Lengerke, C., Skoda, R., & Schroeder, T. (2018). In vitro biomimetic engineering of a human hematopoietic niche with functional properties. Proceedings of the National Academy of Sciences, 115(25), 5688–5695.
Bello, A. B., Park, H., & Lee, S. H. (2018). Current approaches in biomaterial-based hematopoietic stem cell niches. Acta Biomater, 72(3), 1–15.
Braham, M. V. J., Yim, A. S. P. L., Mateos, J. G., Minnema, M. C., Dhert, W. J. A., Oner, F. C., Robin, C., & Alblas, J. (2019). A human hematopoietic niche model supporting hematopoietic stem and progenitor cells in vitro. Advanced Healthcare Materials, 8(10), 235–247.
Choi, J. S., Mahadik, B. P., & Harley, B. A. (2015). Engineering the hematopoietic stem cell niche: Frontiers in biomaterial science. Biotechnology Journal, 10(10), 1529–1545.
Costa, M. H. G., De Soure, A. M., Cabral, J. M. S., Ferreira, F. C., & Da Silva, C. L. (2018). Hematopoietic niche - exploring biomimetic cues to improve the functionality of hematopoietic stem/progenitor cells. Biotechnology Journal, 13(2), 1768–1776.
Danby, R., & Rocha, V. (2015). Clinical use of umbilical cord blood cells. Cord Blood Stem Cells and Regenerative Medicine, 7(12), 77–100.
Darvish, M., Payandeh, Z., Soleimanifar, F., Taheri, B., Soleimani, M., & Islami, M. (2019). Umbilical cord blood mesenchymal stem cells application in hematopoietic stem cells expansion on nanofiber three-dimensional scaffold. Journal of Cellular Biochemistry, 120(7), 12018–12026.
Ferreira, M. S., Jahnen-Dechent, W., Labude, N., Bovi, M., Hieronymus, T., Zenke, M., Schneider, R. K., & Neuss, S. (2012). Cord blood-hematopoietic stem cell expansion in 3D fibrin scaffolds with stromal support. Biomaterials, 33(29), 6987–6997.
Garcia-Abrego, C., Zaunz, S., Toprakhisar, B., Subramani, R., Deschaume, O., Jooken, S., Bajaj, M., Ramon, H., Verfaillie, C., Bartic, C., & Patterson, J. (2020). Towards mimicking the fetal liver niche: The influence of elasticity and oxygen tension on hematopoietic stem/progenitor cells cultured in 3D fibrin hydrogels. International Journal of Molecular Sciences, 21(17), 598–609.
Ge, J., Cai, H., Li, Q., Du, Z., & Tan, W. S. (2013). Effects of telomerase activity and apoptosis on ex vivo expansion of cord blood CD34+ cells. Cell Proliferation, 46(1), 1298–1306.
Guerrouahen, B. S., Ibrahim, A. H., & Rafii, T. A. (2011). Osteoblastic and vascular endothelial niches, their control on normal hematopoietic stem cells, and their consequences on the development of leukemia. Stem Cells International, 2011(1–2), 375857.
Hai-Jiang, W., Xin-Na, D., & Hui-Jun, D. (2008). Expansion of hematopoietic stem/progenitor cells. American Journal of Hematology, 83(12), 922–926.
Huang, X. B., Zhu, B., Wang, X. D., Xiao, R., & Wang, C. S. (2016). Three-dimensional co-culture of mesenchymal stromal cells and differentiated osteoblasts on human bio-derived bone scaffolds supports active multi-lineage hematopoiesis in vitro: Functional implication of the biomimetic HSC niche. International Journal of Molecular Medicine, 38(4), 1141–1151.
Ichida, M., Yui, Y., Yoshioka, K., Tanaka, T., Wakamatsu, T., Yoshikawa, H., & Itoh, K. (2011). Changes in cell migration of mesenchymal cells during osteogenic differentiation. FEBS Letters, 585(24), 4018–4024.
Jobin, C., Cloutier, M., Simard, C., & Néron, S. (2015). Heterogeneity of in vitro-cultured CD34+ cells isolated from peripheral blood. Cytotherapy, 17(10), 1472–1484.
Kim, J. E., Lee, E. J., Wu, Y., Kang, Y. G., & Shin, J.-W. (2019). The combined effects of hierarchical scaffolds and mechanical stimuli on ex vivo expansion of haematopoietic stem/progenitor cells. Artificial Cells Nanomedicine and Biotechnology, 47(1), 586–593.
Ko, T. L., Fu, Y. Y., Shih, Y. H., Lin, Y. H., & Fu, Y. S. (2015). A high efficiency induction of dopaminergic cells from human umbilical mesenchymal stem cells for the treatment of hemiparkinsonian rats. Cell Transplantation, 24(11), 1095–1106.
Lee, D., Kim, D. W., & Cho, J. Y. (2020). Role of growth factors in hematopoietic stem cell niche. Cell Biology Toxicology, 36(2), 131–144.
Leisten, I., Kramann, R., Ferreiraa, M. S. V., Bovi, M., Neuss, S., Ziegler, P., Wagner, W., Knuchel, R., & Schneider, R. K. (2012). 3D co-culture of hematopoietic stem and progenitor cells and mesenchymal stem cells in collagen scaffolds as a model of the hematopoietic niche. Biomaterials, 33(6), 1736–1747.
Lo Iacono, M., Anzalone, R., La Rocca, G., Baiamonte, E., Maggio, A., & Acuto, S. (2017). Wharton's jelly mesenchymal stromal cells as a feeder layer for the ex vivo expansion of hematopoietic stem and progenitor cells: A review. Stem Cell Reviews and Reports, 13(1), 35–49.
Louise, K. (2014). The influence of donor age and in vitro expansion on the proliferation and differentiation properties of donor-matched bone marrow and adipose-derived mesenchymal stem cells: Implications for musculoskeletal tissue engineering. Chemical Journal of Chinese Universities, 35(8), 1658–1664.
Maria, A., Sims, N. A., & Martin, T. J. (2009). What is the true nature of the osteoblastic hematopoietic stem cell niche? Trends in Endocrinology & Metabolism, 26(8), 176–187.
Miyoshi, H., Sato, C., Shimizu, Y., & Morita, M. (2019). Expansion of mouse hematopoietic stem/progenitor cells in three-dimensional cocultures on growth-suppressed stromal cell layer. International Journal of Artificial Organs, 42(7), 374–379.
Mousavi, S. H., Abroun, S., Soleimani, M., & Mowla, S. J. (2018). 3-Dimensional nano-fibre scaffold for ex vivo expansion of cord blood haematopoietic stem cells. Artifical Cells Nanomedicine Biotechnology, 46(4), 740–748.
Pan, X. W., Sun, Q., Cai, H. B., Gao, Y., Tan, W. S., & Zhang, W. A. (2016). Encapsulated feeder cells within alginate beads for ex vivo expansion of cord blood-derived CD34(+) cells. Biomaterials Science, 4(10), 1441–1453.
Pan, X., Sun, Q., Zhang, Y., Cai, H., Gao, Y., Shen, Y., & Zhang, W. (2017). Biomimetic macroporous PCL scaffolds for ex vivo expansion of cord blood-derived CD34(+) cells with feeder cells support. Macromolecular Bioscience, 17(8), 936–944.
Panaroni, C., Tzeng, Y. S., Saeed, H., & Wu, J. Y. (2014). Mesenchymal progenitors and the osteoblast lineage in bone marrow hematopoietic niches. Current Osteoporosis Reports, 12(1), 22–32.
Raic, A., Rodling, L., Kalbacher, H., & Lee-Thedieck, C. (2014). Biomimetic macroporous PEG hydrogels as 3D scaffolds for the multiplication of human hematopoietic stem and progenitor cells. Biomaterials, 35(3), 929–940.
Ribeiro-Filho, A. C., Levy, D., Maria Ruiz, J. L., Mantovani, M. D. C., & Bydlowski, S. P. (2019). Traditional and advanced cell cultures in hematopoietic stem cell studies. Cells, 8(12), 694–705.
Robinson, S. N., Ng, J., Niu, T., Yang, H., McMannis, J. D., Karandish, S., Kaur, I., Fu, P., Del Angel, M., Messinger, R., Flagge, F., de Lima, M., Decker, W., Xing, D., Champlin, R., & Shpall, E. J. (2006). Superior ex vivo cord blood expansion following co-culture with bone marrow-derived mesenchymal stem cells. Bone Marrow Transplantation, 37(4), 359–366.
Rödling, L., Schwedhelm, I., Kraus, S., Bieback, K., Hansmann, J., & Lee-Thedieck, C. (2017). 3D models of the hematopoietic stem cell niche under steady-state and active conditions. Scientific Reports, 7(1), 4625.
Romanov, Y. A., Volgina, N. E., Balashova, E. E., Kabaeva, N. V., Dugina, T. N., & Sukhikh, G. T. (2017). Human umbilical cord mesenchymal stromal cells support viability of umbilical cord blood hematopoietic stem cells but not the "stemness" of their progeny in co-culture. Bulletin of Experimental Biology and Medicine, 163(4), 523–527.
Ruan, J., Wang, X. S., Yu, Z., Wang, Z., Xie, Q., Zhang, D. D., Huang, Y. Z., Zhou, H. F., Bi, X. P., Xiao, C. W., Gu, P., & Fan, X. Q. (2016). Enhanced physiochemical and mechanical performance of chitosan-grafted graphene oxide for superior osteoinductivity. Advanced Functional Materials, 26(7), 1085–1097.
Russell, S., & Taichman, S. G. E. (1994). Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. Brief Definitive Report, 179(5), 1677–1682.
Ryu, K. H., Chun, S., Carbonierre, S., Im, S. A., & Fraser, J. K. (2001). Apoptosis and megakaryocytic differentiation during ex vivo expansion of human cord blood CD34+ cells using thrombopoietin. British Journal of Haematology, 113(2), 470–478.
Severn, C. E., Eissa, A. M., Langford, C. R., Parker, A., Walker, M., Dobbe, J. G. G., Streekstra, G. J., Cameron, N. R., & Toye, A. M. (2019). Ex vivo culture of adult CD34(+) stem cells using functional highly porous polymer scaffolds to establish biomimicry of the bone marrow niche. Biomaterials, 225(8), 2384–2395.
Shrestha, K. R., & Yoo, S. Y. (2019). Phage-based artificial niche: The recent progress and future opportunities in stem cell therapy. Stem Cells International, 2019(10), 696–708.
Sun, Q., Fu, Y., Zhu, X., Tan, W. S., & Cai, H. (2021). Continuous NF-kappaB pathway inhibition promotes expansion of human phenotypical hematopoietic stem/progenitor cells through metabolism regulation. Experimental Cell Research, 399(2), 112468–112468.
Taichman, R. S., & Emerson, S. G. (1998). The role of osteoblasts in the hematopoietic microenvironment. Stem Cells, 16(1), 7–15.
Taichman, R. S., Reilly, M. J., & Emerson, S. G. (1996). Human osteoblasts support human hematopoietic progenitor cells in vitro bone marrow cultures. Blood, 87(2), 518–524.
Wang, M., Chen, B., Sun, X. X., Zhao, X. D., Zhao, Y. Y., Sun, L., Xu, C. G., Shen, B., Su, Z. L., Xu, W. R., & Zhu, W. (2017). Gastric cancer tissue-derived mesenchymal stem cells impact peripheral blood mononuclear cells via disruption of Treg/Th17 balance to promote gastric cancer progression. Experimental Cell Research, 361(1), 19–29.
Wang, J., Zhou, L., Sun, Q., Cai, H., & Tan, W. S. (2020). Porous chitosan derivative scaffolds affect proliferation and osteogenesis of mesenchymal stem cell via reducing intracellular ROS. Carbohydrate Polymers, 237(11), 116108.
Wang, J., Sun, Q., Wei, Y., Hao, M., Tan, W. S., & Cai, H. (2021). Sustained release of epigallocatechin-3-gallate from chitosan-based scaffolds to promote osteogenesis of mesenchymal stem cell. International Journal of Biological Macromolecules, 176(4), 96–105.
Wu, J. Y., Scadden, D. T., & Kronenberg, H. M. (2009). Role of the osteoblast lineage in the bone marrow hematopoietic niches. Journal of Bone Mineral Research, 24(5), 759–764.
Xia, L., & Mcever, R. P. (2014). Stem cells treated by in vitro fucosylation and methods of use. Blood, 80(5), 409–421.
Zhang, S., Ma, B., Wang, S., Duan, J., Qiu, J., Li, D., Sang, Y., Ge, S., & Liu, H. (2018a). Mass-production of fluorescent chitosan/graphene oxide hybrid microspheres for in vitro 3D expansion of human umbilical cord mesenchymal stem cells. Chemical Engineering Journal, 331(7), 675–684.
Zhang, W. Y., Zhang, W. W., Zhang, X., Lu, Q., Cai, H. B., & Tan, W. S. (2018b). Hyperoside promotes ex vivo expansion of hematopoietic stem/progenitor cells derived from cord blood by reducing intracellular ROS level. Process Biochemistry, 72(6), 143–151.
Zhang, P., Zhang, C., Li, J., Han, J., Liu, X., & Yang, H. (2019). The physical microenvironment of hematopoietic stem cells and its emerging roles in engineering applications. Stem Cell Research & Therapy, 10(1), 327–336.
Zhi, W., Deng, L., & Yang, Z. (2007). Role of osteoblasts in the hematopoietic microenvironment of bone marrow and regulatory pathways and mechanisms. Chinese journal of reparative and reconstructive surgery, 21(5), 517–522.
Acknowledgements
This work was supported by the National Key Research and Development Program of China, 2018YFC1105800.
Author information
Authors and Affiliations
Contributions
Jin Wang contributed to the study conception and design. Material preparation, experiments, data collection and analysis were conducted by Jin Wang, Minghao Xiong and Qihao Sun. Flow cytometry analysis and Immunocytochemistry experiments were performed by Jin Wang. The first draft of the manuscript was written by Jin Wang and Haibo Cai, revised by Wen-song Tan and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
There are no conflicts of interest to report.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 162 kb)
Rights and permissions
About this article
Cite this article
Wang, J., Xiong, M., Sun, Q. et al. Three-Dimension Co-culture of Hematopoietic Stem Cells and Differentiated Osteoblasts on Gallic Acid Grafted-Chitosan Scaffold as a Model of Hematopoietic Stem Cells Niche. Stem Cell Rev and Rep 18, 1168–1180 (2022). https://doi.org/10.1007/s12015-021-10325-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-021-10325-5