Abstract
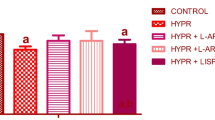
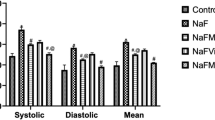
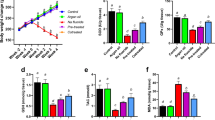
Sodium fluoride (NaF) is one of the neglected environmental pollutants. It is ubiquitously found in the soil, water, and environment. Interestingly, fluoride has been extensively utilized for prevention of dental caries and tartar formation, and may be added to mouthwash, mouth rinse, and toothpastes. This study is aimed at mitigating fluoride-induced hypertension and nephrotoxicity with clofibrate, a peroxisome proliferator–activated receptor-alpha (PPARα) agonist. For this study, forty male Wistar rats were used and randomly grouped into ten rats per group, control, sodium fluoride (NaF; 300 ppm) only, NaF plus clofibrate (250 mg/kg) and NaF plus lisinopril (10 mg/kg), respectively, for 7 days. The administration of NaF was by drinking water ad libitum, while clofibrate and lisinopril were administered by oral gavage. Administration of NaF induced hypertension, and was accompanied with exaggerated oxidative stress; depletion of antioxidant defence system; reduced nitric oxide production; increased systolic, diastolic and mean arterial pressure; activation of angiotensin-converting enzyme activity and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB); and testicular apoptosis. Treatment of rats with clofibrate reduced oxidative stress, improved antioxidant status, lowered high blood pressure through the inhibition of angiotensin-converting enzyme activity, mineralocorticoid receptor over-activation, and abrogated testicular apoptosis. Taken together, clofibrate could offer exceptional therapeutic benefit in mitigating toxicity associated with sodium fluoride.









Similar content being viewed by others
Availability of Data and Materials
Data will be made available based on request from the corresponding author.
References
Abbas M, Siddiqi MH, Khan K, Zahra K, Naqvi AU (2017) Haematological evaluation of sodium fluoride toxicity in Oryctolagus cunniculus. Toxicol Rep 4:450–454. https://doi.org/10.1016/j.toxrep.2017.07.002
Abdelhalim MAK, Qaid HA, Al-Mohy YH, Ghannam MM (2020) The protective roles of vitamin E and α-lipoic acid against nephrotoxicity, lipid peroxidation, and inflammatory damage induced by gold nanoparticles. Int J Nanomedicine 15:729–734. https://doi.org/10.2147/IJN.S192740
Adedara IA, Olabiyi BF, Ojuade TD, Idris UF, Onibiyo EM, Farombi EO (2017) Taurine reverses sodium fluoride-mediated increase in inflammation, caspase-3 activity, and oxidative damage along the brain-pituitary-gonadal axis in male rats. Can J Physiol Pharmacol 95(9):1019–1029. https://doi.org/10.1139/cjpp-2016-0641
Al Dhaybi O, Bakris GL (2020) Non-steroidal mineralocorticoid antagonists: prospects for renoprotection in diabetic kidney disease. Diabetes Obes Metab Suppl 1:69–76. https://doi.org/10.1111/dom.13983
Almeida LF, Tofteng SS, Madsen K, Jensen BL (2020) Role of the renin-angiotensin system in kidney development and programming of adult blood pressure. Clin Sci (Lond) 134(6):641–656. https://doi.org/10.1042/CS20190765
Azushima K, Morisawa N, Tamura K, Nishiyama A (2020) Recent research advances in renin-angiotensin-aldosterone system receptors. Curr Hypertens Rep 22(3):22. https://doi.org/10.1007/s11906-020-1028-6
Bertoluci MC, Cé GV, da Silva AM, Wainstein MV, Boff W, Puñales M (2015) Endothelial dysfunction as a predictor of cardiovascular disease in type 1 diabetes. World J Diabetes 6(5):679–692. https://doi.org/10.4239/wjd.v6.i5.679
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61: 882–888
Boehme AK, Esenwa C, Elkind MS (2017) Stroke risk factors, genetics, and prevention. Circ Res 120(3):472–495. https://doi.org/10.1161/CIRCRESAHA.116.308398
Caimi G, Hopps E, Montana M, Carollo C, Calandrino V, Gallà E, Canino B, Lo Presti R (2020) Behaviour of carbonyl groups in several clinical conditions: analysis of our survey. Clin Hemorheol Microcirc 74(3):299–313. https://doi.org/10.3233/CH-190689
Capelli I, Vitali F, Zappulo F, Martini S, Donadei C, Cappuccilli M, Leonardi L, Girardi A, Aiello V, Galletti S, Faldella G, Poluzzi E, DE Ponti F, Gaetano M (2020) Biomarkers of kidney injury in very-low-birth-weight preterm infants: influence of maternal and neonatal factors. In Vivo 34(3):1333–1339. https://doi.org/10.21873/invivo.11910
Cha Y, Erez T, Reynolds IJ, Kumar D, Ross J, Koytiger G, Kusko R, Zeskind B, Risso S, Kagan E, Papapetropoulos S, Grossman I, Laifenfeld D (2018) Drug repurposing from the perspective of pharmaceutical companies. Br J Pharmacol 175(2):168–180. https://doi.org/10.1111/bph.13798
Chen A, Koehler AN (2020) Transcription factor inhibition: lessons learned and emerging targets. Trends Mol Med 26(5):508–518. https://doi.org/10.1016/j.molmed.2020.01.004
Cheng YY, Rath EM, Linton A, Yuen ML, Takahashi K, Lee K (2020) The current understanding of asbestos-induced epigenetic changes associated with lung cancer. Lung Cancer (Auckl) 11:1–11. https://doi.org/10.2147/LCTT.S186843
Costa PPC, Campos R, Cabral PHB, Gomes VM, Santos CF, Waller SB, de Sousa EHS, Lopes LGF, Fonteles MC, do Nascimento NRF (2020) Antihypertensive potential of cis-[Ru(bpy)2(ImN)(NO)]3+, a ruthenium-based nitric oxide donor. Res Vet Sci 130:153–160. https://doi.org/10.1016/j.rvsc.2020.03.014
Craig A, Mels CMC, Schutte AE, Tsikas D, Kruger R (2020) Nitric oxide-related markers link inversely to blood pressure in black boys and men: the ASOS and African-PREDICT studies. Amino Acids 52(4):639–648. https://doi.org/10.1007/s00726-020-02842-3
Cruz A, Rodríguez-Gómez I, Pérez-Abud R, Vargas MÁ, Wangensteen R, Quesada A, Osuna A, Moreno JM (2011) Effects of clofibrate on salt loading-induced hypertension in rats. J Biomed Biotechnol 2011:469481–469488. https://doi.org/10.1155/2011/469481
da Cost GF, Ognibene DT, da Costa CA, Teixeira MT, Cordeiro VDSC, de Bem GF, Moura AS, Resende AC, de Moura RS (2020) Vitis vinifera L Grape skin extract prevents development of hypertension and altered lipid profile in spontaneously hypertensive rats: role of oxidative stress. Prev Nutr Food Sci 25(1):25–31. https://doi.org/10.3746/pnf.2020.25.1.25
Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R (2003) Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 329(1-2):23–38
Dharmaratne RW (2019) Exploring the role of excess fluoride in chronic kidney disease: a review. Hum Exp Toxicol 38(3):269–279. https://doi.org/10.1177/0960327118814161
Drury RA, Wallington EA (1976) Editors Carlton’s histopathological techniques 4th (ed) Oxford University Press, London, pp 139–142
Ellman GL (1959) Tissue sulfhydryl groups. Arc Biochem Biophy 82: 70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Fan J, Fan X, Guang H, Shan X, Tian Q, Zhang F, Chen R, Ye F, Quan H, Zhang H, Ding L, Gan Z, Xue F, Wang Y, Mao S, Hu L, Gong Y (2020) Upregulation of miR-335-3p by NF-κB transcriptional regulation contributes to the induction of pulmonary arterial hypertension via APJ during hypoxia. Int J Biol Sci 16(3):515–528. https://doi.org/10.7150/ijbs.34517
Fedorova M, Bollineni RC, Hoffmann R (2014) Protein carbonylation as a major hallmark of oxidative damage: update of analytical strategies. Mass Spectrom Rev 33(2):79–97. https://doi.org/10.1002/mas.21381
Feng Z, Liang C, Manthari RK, Wang C, Zhang J (2019) Effects of fluoride on autophagy in mouse Sertoli cells. Biol Trace Elem Res 187(2):499–505. https://doi.org/10.1007/s12011-018-1405-z
Förstermann U, Li H (2011) Therapeutic effect of enhancing endothelial nitric oxide synthase (eNOS) expression and preventing eNOS uncoupling. Br J Pharmacol 164(2):213–223. https://doi.org/10.1111/j.1476-5381.2010.01196.x
Garber JC, Barbee RW, Bielitzki JT, Clayton LA, Donovan JC (2011) The guide for the care and use of laboratory animals, 8th edn. Institute for Laboratory Animal Research. The National Academic Press, Washington DC
Geldsetzer P, Manne-Goehler J, Marcus ME, Ebert C, Zhumadilov Z, Wesseh CS, Tsabedze L, Supiyev A, Sturua L, Bahendeka SK, Sibai AM, Quesnel-Crooks S, Norov B, Mwangi KJ, Mwalim O, Wong-McClure R, Mayige MT, Martins JS, Lunet N, Labadarios D, Karki KB, Kagaruki GB, Jorgensen JMA, Hwalla NC, Houinato D, Houehanou C, Msaidié M, Guwatudde D, Gurung MS, Gathecha G, Dorobantu M, Damasceno A, Bovet P, Bicaba BW, Aryal KK, Andall-Brereton G, Agoudavi K, Stokes A, Davies JI, Bärnighausen T, Atun R, Vollmer S, Jaacks LM (2019) The state of hypertension care in 44 low-income and middle-income countries: a cross-sectional study of nationally representative individual-level data from 1·1 million adults. Lancet 394(10199):652–662. https://doi.org/10.1016/S0140-6736(19)30955-9
Gong DS, Sharma K, Kang KW, Kim DW, Oak MH (2020) Endothelium-dependent relaxation effects of Actinidia arguta extracts in coronary artery: involvement of eNOS/Akt pathway. J Nanosci Nanotechnol 20(9):5381–5384. https://doi.org/10.1166/jnn.2020.17665
Gornal AG, Bardawill JC, David MM (1949) Determination of serum proteins by means of biuret reaction. Res J Biol Chem 177:751–766
Griffin BR, Faubel S, Edelstein CL (2019) Biomarkers of drug-induced kidney toxicity. Ther Drug Monit 41(2):213–226. https://doi.org/10.1097/FTD.0000000000000589
Guizoni DM, Vettorazzi JF, Carneiro EM, Davel AP (2020) Modulation of endothelium-derived nitric oxide production and activity by taurine and taurine-conjugated bile acids. Nitric Oxide 94:48–53. https://doi.org/10.1016/j.niox.2019.10.008
Guo X, Liang XF, Fang L, Yuan X, Zhou Y, He S, Shen D (2015) Effects of lipid-lowering pharmaceutical clofibrate on lipid and lipoprotein metabolism of grass carp (Ctenopharyngodon idellal Val.) fed with the high non-protein energy diets. Fish Physiol Biochem 41(2):331–343. https://doi.org/10.1007/s10695-014-9986-8
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S‑transferases. The first enzymatic step in mercapturic acid formation. J Bio Chem 249:7130–7139
He X, Modi Z, Else T (2020) Hereditary causes of primary aldosteronism and other disorders of apparent excess mineralocorticoid activity. Gland Surg 9(1):150–158. https://doi.org/10.21037/gs.2019.11.20
Hirata Y (2019) Reactive oxygen species (ROS) signaling: regulatory mechanisms and pathophysiological roles. Yakugaku Zasshi 139(10):1235–1241. https://doi.org/10.1248/yakushi.19-00141
Hsu JJ, Lu J, Umar S, Lee JT, Kulkarni RP, Ding Y, Chang CC, Hsiai TK, Hokugo A, Gkouveris I, Tetradis S, Nishimura I, Demer LL, Tintut Y (2018) Effects of teriparatide on morphology of aortic calcification in aged hyperlipidemic mice. Am J Physiol Heart Circ Physiol 314(6):H1203–H1213. https://doi.org/10.1152/ajpheart.00718.2017
Hu Y, Wang Y, Yan T, Feng D, Ba Y, Zhang H, Zhu J, Cheng X, Cui L, Huang H (2019) N-acetylcysteine alleviates fluoride-induced testicular apoptosis by modulating IRE1α/JNK signaling and nuclear Nrf2 activation. Reprod Toxicol 84:98–107. https://doi.org/10.1016/j.reprotox.2019.01.001
Hudnall TW, Chiu CW, Gabbaï FP (2009) Fluoride ion recognition by chelating and cationic boranes. Acc Chem Res 42(2):388–3897. https://doi.org/10.1021/ar8001816
Ibarra-Lara L, Del Valle-Mondragón L, Soria-Castro E, Torres-Narváez JC, Pérez-Severiano F, Sánchez-Aguilar M, Ramírez-Ortega M, Cervantes-Pérez LG, Pastelín-Hernández GS, Oidor-Chan VH, Zarco-Olvera G, Sánchez-Mendoza A (2016) Peroxisome proliferator-activated receptor-alpha stimulation by clofibrate favors an antioxidant and vasodilator environment in a stressed left ventricle. Pharmacol Rep 68(4):692–670. https://doi.org/10.1016/j.pharep.2016.03.002
Ighodaro OM, Akinloye OA (2018) First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx): their fundamental role in the entire antioxidant defence grid. Alexandria J Med 54:287–293
Islam BU, Habib S, Ali SA, Moinuddin Ali A (2017) Role of peroxynitrite-induced activation of poly (ADP-ribose) polymerase (PARP) in circulatory shock and related pathological conditions. Cardiovasc Toxicol 17(4):373–383. https://doi.org/10.1007/s12012-016-9394-7
Jacques-Silva MC, Nogueira CW, LC, Flores , Rocha JBT (2001) Diphenyl diselenide and ascorbic acid changes deposition of selenium and ascorbic acid in liver and brain of mice. Pharmacol 88: 119–125. https://doi.org/10.1034/j.1600-0773.2001.d01-92.x
Jafar TH, Gandhi M, de Silva HA, Jehan I, Naheed A, Finkelstein EA, Turner EL, Morisky D, Kasturiratne A, Khan AH, Clemens JD, Ebrahim S, Assam PN, Feng L (2020) COBRA-BPS Study Group. A community-based intervention for managing hypertension in rural south Asia. N Engl J Med 382(8):717–726. https://doi.org/10.1056/NEJMoa1911965
Jakubczyk K, Dec K, Kałduńska J, Kawczuga D, Kochman J, Janda K (2020) Reactive oxygen species - sources, functions, oxidative damage. Pol Merkur Lekarski 48(284):124–127
Jalili C, Ghanbari A, Roshankhah S, Salahshoor MR (2020) Toxic effects of methotrexate on Rat kidney recovered by crocin as a consequence of antioxidant activity and lipid peroxidation prevention. Iran Biomed J 24(1):39–46
Jollow DJ, Mitchell JR, Zampaglione N (1974) Bromobenzene‑induced liver necrosis Protective role of glutathione and evidence for 3, 4‑bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11(3):151–169. https://doi.org/10.1159/000136485
Kadota Y, Kazama S, Bajotto G, Kitaura Y, Shimomura Y (2012) Clofibrate-induced reduction of plasma branched-chain amino acid concentrations impairs glucose tolerance in rats. JPEN J Parenter Enteral Nutr 36(3):337–343. https://doi.org/10.1177/0148607111414578
Kagawa T, Zárybnický T, Omi T, Shirai Y, Toyokuni S, Oda S, Yokoi T (2019) A scrutiny of circulating microRNA biomarkers for drug-induced tubular and glomerular injury in rats. Toxicol 415:26–36. https://doi.org/10.1016/j.tox.2019.01.011
Karimi A, Radfard M, Abbasi M, Naghizadeh A, Biglari H, Alvani V, Mahdavi M (2018) Fluoride concentration data in groundwater resources of Gonabad, Iran. Data Brief 21:105–110. https://doi.org/10.1016/j.dib.2018.09.062
Kayali R, Cakatay U, Akcay T, Altug T (2006) Effect of alpha-lipoic acid supplementation on markers of protein oxidation in post-mitotic tissues of ageing rat. Cell Biochem Funct 24:79–85. https://doi.org/10.1002/cbf.1190
Kurakova L, Misak A, Tomasova L, Cacanyiova S, Berenyiova A, Ondriasova E, Balis P, Grman M, Ondria K (2020) Mathematical relationships of patterns of 35 rat haemodynamic parameters for conditions of hypertension resulting from decreased nitric oxide bioavailability. Exp Physiol 105(2):312–334. https://doi.org/10.1113/EP088148
Kwon EY, Do GM, Cho YY, Park YB, Jeon SM, Choi MS (2010) Anti-atherogenic property of ferulic acid in apolipoprotein E-deficient mice fed Western diet: comparison with clofibrate. Food Chem Toxicol 48(8-9):2298–2303. https://doi.org/10.1016/j.fct.2010.05.063
Lu YF, Xu YY, Jin F, Wu Q, Shi JS, Liu J (2014) Icariin is a PPARα activator inducing lipid metabolic gene expression in mice. Molecules 19(11):18179–18191. https://doi.org/10.3390/molecules191118179
Lussi A, Buzalaf MAR, Duangthip D, Anttonen V, Ganss C, João-Souza SH, Baumann T, Carvalho TS (2019) The use of fluoride for the prevention of dental erosion and erosive tooth wear in children and adolescents. Eur Arch Paediatr Dent 20(6):517–527. https://doi.org/10.1007/s40368-019-00420-0
Macchiavello S, Fardella C, Baudrand R (2019) Update in the clinical management of low renin hypertension. Rev Med Chil 147(4):490–498. https://doi.org/10.4067/S0034-98872019000400490
Mesdaghinia A, Vaghefi KA, Montazeri A, Mohebbi MR, Saeedi R (2010) Monitoring of fluoride in groundwater resources of Iran. Bull Environ Contam Toxicol 84(4):432–447. https://doi.org/10.1007/s00128-010-9950-y
Mills KT, Stefanescu A, He J (2020) The global epidemiology of hypertension. Nat Rev Nephrol 16(4):223–237. https://doi.org/10.1038/s41581-019-0244-2
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175. https://doi.org/10.1007/s12011-021-02722-1
More VR, Campos CR, Evans RA, Oliver KD, Chan GN, Miller DS, Cannon RE (2017) PPAR-α, a lipid-sensing transcription factor, regulates blood-brain barrier efflux transporter expression. J Cereb Blood Flow Metab 37(4):1199–1212. https://doi.org/10.1177/0271678X16650216
Oktaviono YH, Kusumawardhani N (2020) Hyperkalemia associated with angiotensin converting enzyme inhibitor or angiotensin receptor blockers in chronic kidney disease. Acta Med Indones 52(1):74–79
Olaleye SB, Adaramoye OA, Erigbali PP, Adeniyi OS (2007) Lead exposure increases oxidative stress in the gastric mucosa of HCl/ethanol exposed rats. World J Gastroenterol 13:5121–5126. https://doi.org/10.3748/wjg.v13.i38.5121
Ou M, Zhao H, Ji G, Zhao X, Zhang Q (2020) Long noncoding RNA MALAT1 contributes to pregnancy-induced hypertension development by enhancing oxidative stress and inflammation through the regulation of the miR-150-5p/ET-1 axis. FASEB J 34(5):6070–6085. https://doi.org/10.1096/fj.201902280R
Oyagbemi AA, Omobowale TO, Asenuga ER, Adejumobi AO, Ajibade TO, Ige TM, Ogunpolu BS, Adedapo AA, Yakubu MA (2017) Sodium fluoride induces hypertension and cardiac complications through generation of reactive oxygen species and activation of nuclear factor kappa beta. Environ Toxicol 32(4):1089–1101. https://doi.org/10.1002/tox.22306
Oyagbemi AA, Omobowale TO, Ola-Davies OE, Asenuga ER, Ajibade TO, Adejumobi OA, Afolabi JM, Ogunpolu BS, Falayi OO, Saba AB, Adedapo AA, Yakubu MA (2018a) Luteolin-mediated Kim-1/NF-kB/Nrf2 signaling pathways protects sodium fluoride-induced hypertension and cardiovascular complications. Biofactors 44(6):518–531. https://doi.org/10.1002/biof.1449
Oyagbemi AA, Omobowale TO, Ola-Davies OE, Asenuga ER, Ajibade TO, Adejumobi OA, Afolabi JM, Ogunpolu BS, Falayi OO, Ayodeji F, Hassan FO, Saba AB, Adedapo AA, Yakubu MA (2018b) Ameliorative effect of rutin on sodium fluoride-induced hypertension through modulation of Kim-1/NF-κB/Nrf2 signaling pathway in rats. Environ Toxicol 33(12):1284–1297. https://doi.org/10.1002/tox.22636
Oyagbemi AA, Omobowale TO, Ola-Davies OE, Asenuga ER, Ajibade TO, Adejumobi OA, Arojojoye OA, Afolabi JM, Ogunpolu BS, Falayi OO, Hassan FO, Ochigbo GO, Saba AB, Adedapo AA, Yakubu MA (2018c) Quercetin attenuates hypertension induced by sodium fluoride via reduction in oxidative stress and modulation of HSP 70/ERK/PPARγ signaling pathways. Biofactors 44(5):465–479. https://doi.org/10.1002/biof.1445
Oyagbemi AA, Omobowale TO, Awoyomi OV, Ajibade TO, Falayi OO, Ogunpolu BS, Okotie UJ, Asenuga ER, Adejumobi OA, Hassan FO, Ola-Davies OE, Saba AB, Adedapo AA, Yakubu MA (2019) Cobalt chloride toxicity elicited hypertension and cardiac complication via induction of oxidative stress and upregulation of COX-2/Bax signaling pathway. Hum Exp Toxicol 38(5):519–532. https://doi.org/10.1177/0960327118812158
Oyagbemi AA, Adebiyi OE, Adigun KO, Ogunpolu BS, Falayi OO, Hassan FO, Folarin OR, Adebayo AK, Adejumobi OA, Asenuga ER, Ola-Davies OE, Omobowale TO, Olopade JO, Saba AB, Adedapo AA, Nkadimeng SM, McGaw LJ, Oguntibeju OO, Yakubu MA (2020) Clofibrate, a PPAR-α agonist, abrogates sodium fluoride-induced neuroinflammation, oxidative stress, and motor incoordination via modulation of GFAP/Iba-1/anti-calbindin signaling pathways. Environ Toxicol 35(2):242–253. https://doi.org/10.1002/tox.22861
Pasala S, Carmody JB (2017) How to use serum creatinine, cystatin C and GFR. Arch Dis Child Educ Pract Ed 102(1):37–43. https://doi.org/10.1136/archdischild-2016-311062
Perera T, Ranasinghe S, Alles N, Waduge R (2018) Effect of fluoride on major organs with the different time of exposure in rats. Environ Health Prev Med 23(1):17. https://doi.org/10.1186/s12199-018-0707-2
Pilic L, Pedlar CR, Mavrommatis Y (2016) Salt-sensitive hypertension: mechanisms and effects of dietary and other lifestyle factors. Nutr Rev 74(10):645–658. https://doi.org/10.1093/nutrit/nuw028
Poetsch AR (2020) The genomics of oxidative DNA damage, repair, and resulting mutagenesis. Comput Struct Biotechnol J 18:207–219. https://doi.org/10.1016/j.csbj.2019.12.013
Puar YR, Shanmugam MK, Fan L, Arfuso F, Sethi G, Tergaonkar V (2018) Evidence for the involvement of the master transcription factor NF-κB in cancer initiation and progression. Biomedicines 6(3):E82. https://doi.org/10.3390/biomedicines6030082
Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, Doig A, Guilliams T, Latimer J, McNamee C, Norris A, Sanseau P, Cavalla D, Pirmohamed M (2019) Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov 18(1):41–58. https://doi.org/10.1038/nrd.2018.168
Reznick AZ, Packer L (1994) Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol 233:357–363. https://doi.org/10.1016/s0076-6879(94)33041-7
Ribeiro SC, Figueiredo AE, Barretti P, Pecoits-Filho R, de Moraes TP (2017) BRAZPD Investigators. Impact of renin-angiotensin aldosterone system inhibition on serum potassium levels among peritoneal dialysis patients. Am J Nephrol 46(2):150–155. https://doi.org/10.1159/000479011
Sankaralingam S, Desai KM, Wilson TW (2008) Chronic clofibrate administration prevents saline-induced endothelial dysfunction and oxidative stress in young Sprague-Dawley rats. Clin Invest Med 31(2):E62–E70. https://doi.org/10.25011/cim.v31i2.3365
Saura M, Marquez S, Reventun P, Olea-Herrero N, Arenas MI, Moreno-Gómez-Toledano R, Gómez-Parrizas M, Muñóz-Moreno C, González-Santander M, Zaragoza C, Bosch RJ (2014) Oral administration of bisphenol A induces high blood pressure through angiotensin II/CaMKII-dependent uncoupling of eNOS. FASEB J 28(11):4719–4728. https://doi.org/10.1096/fj.14-252460
Shenoy PS, Sen U, Kapoor S, Ranade AV, Chowdhury CR, Bose B (2019) Sodium fluoride induced skeletal muscle changes: degradation of proteins and signaling mechanism. Environ Pollut 244:534–548. https://doi.org/10.1016/j.envpol.2018.10.034
Soveri I, Helmersson-Karlqvistm J, Fellström B, Larsson A (2020) Day-to-day variation of the kidney proximal tubular injury markers urinary cystatin C, KIM1, and NGAL in patients with chronic kidney disease. Ren Fail 42(1):400–404. https://doi.org/10.1080/0886022X.2020.1757463
Tam LM, Price NE, Wang Y (2020) Molecular mechanisms of arsenic-induced disruption of DNA repair. Chem Res Toxicol 33(3):709–726. https://doi.org/10.1021/acs.chemrestox.9b00464
Tian J, Popal MS, Huang R, Zhang M, Zhao X, Zhang M, Song X (2020) Caveolin as a novel potential therapeutic target in cardiac and vascular diseases: a mini review. Aging Dis 11(2):378–389. https://doi.org/10.14336/AD.2019.09603
Udupa V, Prakash V (2018) Gentamicin induced acute renal damage and its evaluation using urinary biomarkers in rats. Toxicol Rep 6:91–99. https://doi.org/10.1016/j.toxrep.2018.11.015
Ulker E, Parker WH, Raj A, Qu ZC, May JM (2016) Ascorbic acid prevents VEGF-induced increases in endothelial barrier permeability. Mol Cell Biochem 412(1-2):73–79. https://doi.org/10.1007/s11010-015-2609-6
Varshney R, Kale RK (1990) Effect of calmodulin antagonists on radiation induced lipid peroxidation in microsomes. Int J Radiat Biol 58:733–743. https://doi.org/10.1080/09553009014552121
Wan L, Liu J, Huang CB, Sun Y, Zhang XJ, Liu TY (2020) Effect of triptolide on cardiac function in arthritic rats and its mechanism. Sichuan Da Xue Xue Bao Yi Xue Ban 51(2):207–212. https://doi.org/10.12182/20200360602
Wang D, Liu B, Tao W, Hao Z, Liu M (2015) Fibrates for secondary prevention of cardiovascular disease and stroke. Cochrane Database Syst Rev 10:CD009580. https://doi.org/10.1002/14651858.CD009580.pub2
Wang H, Varagic J, Nagata S, Kon ND, Ahmad S, VonCannon JL, Wright KN, Sun X, Deal D, Groban L, Ferrario CM (2020) Differential expression of the angiotensin-(1-12)/chymase axis in human atrial tissue. J Surg Res 253:173–184. https://doi.org/10.1016/j.jss.2020.03.051
Wolff SF (1994) Ferrous ion oxidation in the presence of ferric ion indicator xylenol orange for measurement of hydrogen peroxides. Methods Enzymol 233:182–189. https://doi.org/10.1016/S0076-6879(94)33021-2
Xie YD, Chen ZZ, Shao LH, Wang QT, Li N, Lu WF, Xu YH, Gao YQ, Guo LY, Liu HL, Li YP, Yang GD, Bian XL (2018) A new multifunctional hydroxytyrosol-clofibrate with hypolipidemic, antioxidant, and hepatoprotective effects. Bioorg Med Chem Lett 28(18):3119–3122. https://doi.org/10.1016/j.bmcl.2018.06.010
Xiong Y, Aroor AR, Ramirez-Perez F, Jia G, Habibi J, Manrique-Acevedo C, Lastra G, Chen D, DeMarco V, Martinez-Lemus LA, Hill MA, Jaisser F, Sowers JR, Whaley-Connell A (2020) Western diet induces renal artery endothelial stiffening that is dependent on the epithelial Na+ channel. Am J Physiol Renal Physiol 318(5):F1220–F1228. https://doi.org/10.1152/ajprenal.00517.2019
Yi W, Fu P, Fan Z, Aso H, Tian C, Meng Y, Liu J, Yamori Y, Nara Y, Ying C (2010) Mitochondrial HMG-CoA synthase partially contributes to antioxidant protection in the kidney of stroke-prone spontaneously hypertensive rats. Nutrition 26(11-12):1176–1180. https://doi.org/10.1016/j.nut.2009.10.010
Yoshida S, Ishizawa K, Ayuzawa N, Ueda K, Takeuchi M, Kawarazaki W, Fujita T, Nagase M (2014) Renin inhibition ameliorates renal damage through prominent suppression of both angiotensin I and II in human renin angiotensinogen transgenic mice with high salt loading. Clin Exp Nephrol 18(4):593–599. https://doi.org/10.1007/s10157-013-0893-6
Younas A, Mushtaq N, Khattak JA, Javed T, Rehman HU, Farooqi A (2019) High levels of fluoride contamination in groundwater of the semi-arid alluvial aquifers, Pakistan: evaluating the recharge sources and geochemical identification via stable isotopes and other major elemental data. Environ Sci Pollut Res Int 26(35):35728–35741. https://doi.org/10.1007/s11356-019-06610-z
Younus H (2018) Therapeutic potentials of superoxide dismutase. Int J Health Sci (Qassim) 12(3):88–93
Yousefipour Z, Newaz M (2014) PPARα ligand clofibrate ameliorates blood pressure and vascular reactivity in spontaneously hypertensive rats. Acta Pharmacol Sin 35(4):476–482. https://doi.org/10.1038/aps.2013.193
Zhang S, Jiang C, Liu H, Guan Z, Zeng Q, Zhang C, Lei R, Xia T, Gao H, Yang L, Chen Y, Wu X, Zhang X, Cui Y, Yu L, Wang Z, Wang A (2013) Fluoride-elicited developmental testicular toxicity in rats: roles of endoplasmic reticulum stress and inflammatory response. Toxicol Appl Pharmacol 271(2):206–215. https://doi.org/10.1016/j.taap.2013.04.033
Zhang S, Niu Q, Gao H, Ma R, Lei R, Zhang C, Xia T, Li P, Xu C, Wang C, Chen J, Dong L, Zhao Q, Wang A (2016) Excessive apoptosis and defective autophagy contribute to developmental testicular toxicity induced by fluoride. Environ Pollut 212:97–104. https://doi.org/10.1016/j.envpol.2016.01.059
Zhu A, Yoneda T, Demura M, Karashima S, Usukura M, Yamagishi M, Takeda Y (2009) Effect of mineralocorticoid receptor blockade on the renal renin-angiotensin system in Dahl salt-sensitive hypertensive rats. J Hypertens 27(4):800–805. https://doi.org/10.1097/HJH.0b013e328325d861
Ziaee M, Samini M, Bolourtchian M, Ghaffarzadeh M, Ahmadi M, Egbal MA, Khorrami A, Andalib S, Maleki-Dizaji N, Garjani A (2012) Synthesis of a novel siliconized analog of clofibrate (silafibrate) and comparison of their anti-inflammatory activities. Iran J Pharm Res 11(1):91–95
Acknowledgements
The authors are grateful to Cape Peninsula University of Technology and National Research Foundation (South Africa) for financial support granted to Prof. OO Oguntibeju.
Author information
Authors and Affiliations
Contributions
Conceptualization: Ademola Adetokunbo Oyagbemi
Methodology: Blessing Seun Ogunpolu, Fasilat Oluwakemi Hassan, Olufunke Olubunmi Falayi, and Iyanuoluwa Omolola Ogunmiluyi
Formal analysis and investigation: Olumuyiwa Abiola Adejumobi, Theophilus Aghogho Jarikre, Olumide Samuel Ajani, Ebunoluwa Racheal Asenuga, Idayat Titilayo Gbadamosi, Aduragbenro Deborah A. Adedapo, Abimbola Obemisola Aro
Writing—original draft preparation: Ademola Adetokunbo Oyagbemi
Writing—review and editing: Ademola Adetokunbo Oyagbemi, Sanah Malomile Nkadimeng, Lyndy Joy McGaw, and Prudence Ngalula Kayoka-Kabongo
Funding acquisition: Temidayo Olutayo Omobowale, Oluwatosin Adetola Arojojoye, Oluwafemi Omoniyi Oguntibeju and Momoh Audu Yakubu
Resources: Oluwafemi Omoniyi Oguntibeju and Momoh Audu Yakubu
Supervision: Olufunke Eunice Ola-Davies, Adebowale Benard Saba, Adeolu Alex Adedapo, Benjamin Obukowho Emikpe, Matthew Olugbenga Oyeyemi
Corresponding author
Ethics declarations
Ethics Approval
All animals were treated in accordance with Guide for the Care and Use of Laboratory Animals (8th edition, National Academies Press). The Animal Care and Use Research Ethical Committee of the Faculty of Veterinary Medicine of Ibadan approved the study with ethical approval number UI-ACUREC/19/124.
Consent to Participate
Not applicable
Consent for Publication
Not applicable
Conflicts of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oyagbemi, A.A., Adejumobi, O.A., Jarikre, T.A. et al. Clofibrate, a Peroxisome Proliferator–Activated Receptor-Alpha (PPARα) Agonist, and Its Molecular Mechanisms of Action against Sodium Fluoride–Induced Toxicity. Biol Trace Elem Res 200, 1220–1236 (2022). https://doi.org/10.1007/s12011-021-02722-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02722-1