Abstract
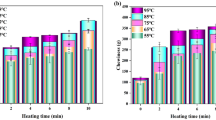
Protein denaturation is the main reason for physicochemical changes in muscle foods during thermal processing. The purpose of this study was to understand the kinetics of quality degradation and protein denaturation of Atlantic salmon (Salmo salar) during thermal pasteurization. Findings indicate that cook loss, area shrinkage, and protein denaturation parameters (both dielectric loss and differential scanning calorimetry (DSC) studies) were best fitted to a first-order reaction. In addition, color parameters followed zero-order kinetics. Fourier transform infrared spectroscopy (FTIR) results showed that increasing heating time and temperature reduced the α-helix peak, indicating protein denaturation. At the same time, the β-sheet peak intensity increased, which is related to protein aggregation. Correlation loading plots from FTIR spectra showed that protein denaturation was dominant below 75 °C. Above this transition temperature, protein denaturation occurred rapidly and protein aggregation became more obvious. The dielectric properties in cooked salmon at different times and temperature increased with increasing heating time and temperature, while penetration depth decreased significantly (P < 0.05). The kinetics of protein denaturation from DSC showed that the activation energy of 300.6 kJ/mol and k 0 was 1.1 × 1042 min−1. Our findings on kinetic data from protein denaturation and physical changes may be used to improve the processing schedule in the production of safe, high-quality pasteurized salmon.










Similar content being viewed by others
References
Ahmed, J., Ramaswamy, H. S., & Raghavan, G. S. V. (2008). Dielectric properties of soybean protein isolate dispersions as a function of concentration, temperature and pH. LWT-Food Science and Technology, 41, 71–81.
Astruc, T., Peyrin, F., Venien, A., Labas, R., Abrantes, M., Dumas, P., & Jamme, F. (2012). In situ thermal denaturation of myofibre sub-type proteins studied by immunohistofluorescence and synchrotron radiation FT-IR microspectroscopy. Food Chemistry, 134, 1044–1051.
Basaran, P., Basaran-Akgul, N., & Rasco, B. (2010). Dielectric properties of chicken and fish muscle treated with microbial transglutaminase. Food Chemistry, 120, 361–370.
Barbera, S., & Tassone, S. (2006). Meat cooking shrinkage: measurement of a new meat quality parameter. Meat Science, 73(3), 467–474.
Barringer, S. A., Fleischmann, A. M., Davis, E. A., & Gordon, J. (1995). The dielectric properties of whey protein as indicators of change in polymer mobility. Food Hydrocolloids, 9, 343–348.
Bertola, N. C., Bevilacqua, A. E., & Zaritzky, N. E. (1994). Heat treatment effect on texture changes and thermal denaturation of proteins in beef muscle. Journal of Food Processing and Preservation, 18, 31–46.
Bertram, H. C., Kohler, A., Bocker, U., Ofstad, R., & Andersen, H. J. (2006). Heat-induced changes in myofibrillar protein structure and myowater of two pork qualities. A combined FT-IR spectroscopy and low-field NMR relaxometry study. Journal of Agricultural and Food Chemistry, 54, 1740–1746.
Bhattacharya, S., Choudhury, G. S., & Studebaker, S. (1994). Color changes during thermal processing of Pacific chum salmon. Journal of Aquatic Food Product Technology, 3(1), 39–48.
Bircan, C., & Barringer, S. A. (2002). Use of dielectric properties to detect egg protein denaturation. Journal of Microwave Power and Electromagnetic Energy, 37, 90–96.
Bocker, U., Kohler, A., Aursand, I. G., & Ofstad, R. (2008). Effects of brine salting with regards to raw material variation of Atlantic salmon (Salmo salar) muscle investigated by Fourier transform infrared microspectroscopy. Journal of Agricultural and Food Chemistry, 56, 5129–5137.
Brunton, N. P., Lyng, J. G., Zhang, L., & Jacquier, J. C. (2006). The use of dielectric properties and other physical analyses for assessing protein denaturation in beef Biceps femoris muscle during cooking from 5 to 85°C. Meat Science, 72, 236–244.
Buffler, C. R. (Ed.). (1993). Microwave cooking and processing (pp. 47–68). New York: Van Nostrand Reinhold.
Cao, L., Rasco, B., Tang, J., Niu, L., Lai, K., Fan, Y., & Huang, Y. (2016). Effects of freshness on the cool loss and shrinkage of grass carp (Ctenopharyngodon idellus) fillets following pasteurization. International Journal of Food Properties, 19, 2297–2306.
Carton, I., Bocker, U., Ofstad, R., Sorheim, O., & Kohler, A. (2009). Monitoring secondary structural changes in salted and smoked salmon muscle myofiber proteins by FT-IR microspectroscopy. Journal of Agricultural and Food Chemistry, 57, 3563–3570.
Clark, A. H. (1998). Gelation of globular proteins. In S. E. Hill, D. A. Ledward, & J. R. Mitchell (Eds.), Functional properties of food macromolecules (2nd ed., pp. 77–142). Gaithersburh: Aspen Publisher.
Dima, J. B., Barón, P. J., & Zaritzky, N. E. (2012). Mechanical modeling of the heat transfer process and protein denaturation during the thermal treatment of Patagonian marine crabs. Journal of Food Engineering, 113, 623–634.
FAO (2014). Atlantic salmon production statistics. http://www.fao.org/fishery/species/2929/en.
Gaze, J. E., Boyd, A. R., & Shaw, H. L. (2006). Heat inactivation of Listeria monocytogenes Scott A on potato surfaces. Journal of Food Engineering, 76, 27–31.
Gibson, K. E., & Schwab, K. J. (2011). Thermal inactivation of human norovirus surrogates. Food and Environmental Virology, 3, 74–77.
Grinberg, V. Y., Burova, T. V., Haertl, T., & Tolstoguzov, V. B. (2000). Interpretation of DSC data on protein denaturation complicated by kinetic and irreversible effects. Journal of Biotechnology, 79, 269–280.
Guan, D., Cheng, M., Wang, Y., & Tang, J. (2004). Dielectric properties of mashed potatoes relevant to microwave and radio-frequency pasteurization and sterilization processes. Journal of Food Science, 69(1), 30–37.
Haard, N. F. (1992). Biochemistry and chemistry of color and color changes in seafoods. In G. J. Flick & R. E. Martin (Eds.), Advances in seafood biochemistry: composition and quality (pp. 305–360). Lancaster, PA: Technomic Publishing Company Inc..
Jantakoson, T., Kijroongrojana, K., & Benjakul, S. (2012). Effect of high pressure and heat treatments on black tiger shrimp (Penaeus monodon Fabricius) muscle protein. International Aquatic Research, 4, 19. doi:10.1186/2008-6970-4-19.
Khaldi, M., Ronse, G., André, C., Blanpain-Avet, P., Bouvier, L., Six, T., Bornaz, S., Croguennec, T., Jeantet, R., & Delaplace, G. (2015). Denaturation kinetics of whey protein isolate solutions and fouling mass distribution in a plate heat exchanger. International Journal of Chemical Engineering. doi:10.1155/2015/139638.
Kong, F., Tang, J., Rasco, B., & Crapo, C. (2007a). Kinetics of salmon quality changes during thermal processing. Journal of Food Engineering, 83, 510–520.
Kong, F., Tang, J., Rasco, B., Crapo, C., & Smiley, S. (2007b). Quality changes of salmon (Oncorhynchus gorbuscha) muscle during thermal processing. Journal of Food Science, 72(2), 103–111.
Kirschner, C., Ofstad, R., Skarpeid, H. J., Host, V., & Kohler, A. (2004). Monitoring of denaturation processes in aged beef loin by Fourier transform infrared microspectroscopy. Journal of Agricultural and Food Chemistry, 52, 3920–3929.
Ling, B., Tang, J., Kong, F., Mitcham, E. J., & Wang, S. (2015). Kinetics of food quality changes during thermal processing: a review. Food and Bioprocess Technology, 8, 343–358.
Ma, C., & Harwalkar, V. R. (1991). Thermal analysis of food proteins. Advances in Food and Nutrition Research, 35, 317–366.
Mao, W., Li, X., Fukuoka, M., Liu, S., Ji, H., & Sakai, N. (2016). Study of Ca2+-ATPase activity and solubility in the whole Kuruma prawn (Marsupenaeus japonicas) meat during heating: based on the kinetics analysis of myofibril protein thermal denaturation. Food and Bioprocess Technology, 9(9), 1511–1520.
Niu, L., Rasco, B., Tang, J., Lai, K., & Huang, Y. (2015). Relationship of changes in quality attributes and protein solubility of ground beef under pasteurization conditions. LWT-Food Science and Technology. doi:10.1016/j.lwt.2014.11.016.
Ofstad, R., Kidman, S., & Hermansson, A. M. (1996). Ultramicroscopical structures and liquid loss in heated cod (Gadus morhua) and salmon (Salmo salar) muscle. Journal of the Science of Food and Agriculture, 72(3), 337–347.
Ojagh, S. M., Nunez-Flores, R., Lopez-Caballero, M. E., Montero, M. P., & Gomez-Guillen, M. C. (2011). Lessening of high pressure induced changes in Atlantic salmon muscle by the combined use of a fish gelatin-lignin film. Food Chemistry, 125, 595–606.
Ovissipour, M., Rasco, B., Tang, J., & Sablani, S. S. (2013). Kinetics of quality changes in whole blue mussel (Mytilus edulis) during pasteurization. Food Research International, 53, 141–148.
Palka, K., & Daun, H. (1999). Changes in texture, cooking losses, and myofibrillar structure of bovine M. semitendinosus during heating. Meat Science, 51(3), 237–243.
Paredi, M. E., Tomas, M. C., Añon, M. C., & Crupkin, M. (1998). Thermal stability of myofibrillar protein from smooth and striated muscles of scallop (Chlamys tehuelchus): a differential scanning calorimetric study. Journal of Agricultural and Food Chemistry, 46, 3971–3976.
Rattanasatheirn, N., Benjakul, S., Visessanguan, W., & Kijroongrojana, K. (2008). Properties, translucence, and microstructure of Pacific White shrimp treated with mixed phosphates as affected by freshness and deveining. Journal of Food Science, 73(1), 31–40.
Skipnes, D., Ostby, M. L., & Hendrickx, M. E. (2007). A method for characterising cook loss and water holding capacity in heat treated cod (Gadus morhua) muscle. Journal of Food Engineering, 80, 1078–1085.
Skipnes, D., Van der Plancken, I., Van Loey, A., & Hendrickx, M. (2008). Kinetics of heat denaturation of proteins from farmed Atlantic cod (Gadus mohua). Journal of Food Engineering, 85(1), 51–58.
Skipnes, D., Johnson, S. O., Skara, T., Sivertsik, M., & Lekang, O. (2011). Optimization of heat processing of farmed Atlantic cod (Gadus morhua) muscle with respect of cook loss, water holding capacity, color, and texture. Journal of Aquatic Food Product Technologies, 20, 331–340.
Verhaeghe, T., Vlaemynck, G., Block, J. D., Van Weyenberg, S., Braeckman, R., & Hendricks, M. (2016). Kinetics of heat induced muscle protein denaturation of brown shrimp (Crangon crangon). Journal of Food Engineering, 191, 88–94.
Wang, Y., Wig, T. D., Tang, J., & Hallberg, L. M. (2003). Dielectric properties of foods relevant to RF and microwave pasteurization and sterilization. Journal of Food Engineering, 57, 257–268.
Wang, Y., Tang, J., Rasco, B., Kong, F., & Wang, S. (2008). Dielectric properties of salmon fillets as a functional of temperature and composition. Journal of Food Engineering, 87, 2367–2246.
Wang, J., Tang, J., Wang, Y., & Swanson, B. (2009). Dielectric properties of egg whites and whole eggs as influenced by thermal treatments. LWT-Food Science and Technology, 42, 1204–1212.
Whistler, R. L., & Daniel, J. R. (1985). Carbohydrates. In O. R. Fennema (Ed.), Food chemistry (pp. 98–99). Marcel Dekker.
Wolz, M., & Kulozik, U. (2015). Thermal denaturation kinetics of whey proteins at high protein concentrations. International Dairy Journal, 49, 95–101.
Wu, Z., Bertram, H. C., Kohler, A., Bocker, U., Ofstad, R., & Andersen, H. J. (2006). Influence of aging and salting on protein secondary structures and water distribution in uncooked and cooked pork. A combined FT-IR microspectroscopy and 1H NMR relaxometry study. Journal of Agricultural and Food Chemistry, 54, 8589–8597.
Yan, Y.-B., Wang, Q., He, H.-W., & Zhou, H.-M. (2004). Protein thermal aggregation involves distinct regions: sequential events in the heat-induced unfolding and aggregation of hemoglobin. Biophysical Journal, 86, 1682–1690.
Yoon, W. B., Gunasekaran, S., & Park, J. W. (2004). Characterization of thermorheological behavior of Alaska Pollock and Pacific Whiting surimi. Journal of Food Science, 69(7), 338–343.
Acknowledgements
This study was partially funded by the following grants: USDA-NIFA 2011-68003-20096, USDA-NIFA 2016-68003-24840, as well as the Agricultural Research Center at Washington State University. The authors would like to thank Dr. Setareh Shiroodi at UC Davis, California; Dr. Kanishka Bhunia, Dr. Huimin Lin, Mr. Frank Loring Younce, and Mr. Stewart Bohnet at Washington State University, Pullman, Washington; and Dr. Xiaonan Lu and Ms. Yaxi Hu at the University of British Columbia, Vancouver, Canada for technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ovissipour, M., Rasco, B., Tang, J. et al. Kinetics of Protein Degradation and Physical Changes in Thermally Processed Atlantic Salmon (Salmo salar). Food Bioprocess Technol 10, 1865–1882 (2017). https://doi.org/10.1007/s11947-017-1958-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-017-1958-4