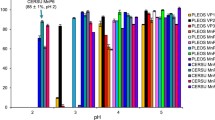
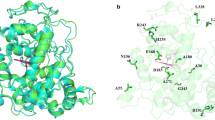
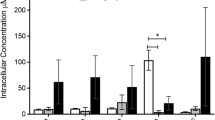
Abstract
The enzymatic degradation of azo dyes is a promising alternative to ineffective chemical and physical remediation methods. Lignin peroxidase (LiP) from Phanerochaete chrysosporium is a heme-containing lignin-degrading oxidoreductase that catalyzes the peroxide-dependent oxidation of diverse molecules, including industrial dyes. This enzyme is therefore ideal as a starting point for protein engineering. Accordingly, we subjected two positions (165 and 264) in the environment of the catalytic Trp171 residue to saturation mutagenesis, and the resulting library of 104 independent clones was expressed on the surface of yeast cells. This yeast display library was used for the selection of variants with the ability to break down structurally-distinct azo dyes more efficiently. We identified mutants with up to 10-fold greater affinity than wild-type LiP for three diverse azo dyes (Evans blue, amido black 10B and Guinea green) and up to 13-fold higher catalytic activity. Additionally, cell wall fragments displaying mutant LiP enzymes were prepared by toluene-induced cell lysis, achieving significant increases in both enzyme activity and stability compared to a whole-cell biocatalyst. LiP-coated cell wall fragments retained their initial dye degradation activity after 10 reaction cycles each lasting 8 h. The best-performing mutants removed up to 2.5-fold more of each dye than the wild-type LiP in multiple reaction cycles.

Similar content being viewed by others
References
Amin K A, Abdel Hameid H II, Abd Elsttar A H (2010). Effect of food azo dyes tartrazine and carmoisine on biochemical parameters related to renal, hepatic function and oxidative stress biomarkers in young male rats. Food and Chemical Toxicology, 48(10): 2994–2999
Blazić M, Kovačević G, Prodanović O, Ostafe R, Gavrović-Jankulović M, Fischer R, Prodanović R (2013). Yeast surface display for the expression, purification and characterization of wild-type and B11 mutant glucose oxidases. Protein Expression and Purification, 89(2): 175–180
Blodig W, Smith A T, Doyle W A, Piontek K (2001). Crystal structures of pristine and oxidatively processed lignin peroxidase expressed in Escherichia coli and of the W171F variant that eliminates the redox active tryptophan 171. Implications for the reaction mechanism. Journal of Molecular Biology, 305(4): 851–861
Boder E T, Wittrup K D (1997). Yeast surface display for screening combinational polypeptide libraries. Nature Biotechnology, 15(6): 553–557
Boder E T, Wittrup K D (2000). Yeast surface display for directed evolution of protein expression, affinity and stability. Methods in Enzymology, 328(4): 430–444
Chequer F M D, Angeli J P F, Ferraz E R A, Tsuboy M S, Marcarini, J C, Mantovani M S, Oliveira D O (2009). The azo dyes Disperse Red 1 and Disperse Orange 1 increase the micronuclei frequencies in human lymphocytes and in HepG2 cells. Mutant Research/Genetic Toxicology and Environmental Mutagenesis, 676(1–2): 83–86
Cherf G M, Cochran J R (2015). Applications of yeast surface display for protein engineering. Methods in Molecular Biology (Clifton, N.J.), 1319: 155–175
Chu F K, Maley F (1980). The effect of glucose on the synthesis and glycosylation of the polypeptide moiety of yeast external invertase. Journal of Biological Chemistry, 255(13): 6392–6397
Datta S, Christena L R, Rajaram Y R S (2013). Enzyme immobilization: An overview on techniques and support materials. Biotechnology (Faisalabad), 3(1): 1–9
Fernández-Fueyo E, Ruiz-Dueñas F J, Martínez AT (2014). Engineering a fungal peroxidase that degrades lignin at very acidic pH. Biotechnology for Biofuels, 7(1): 114–126
Gai S A, Wittrup K D (2007). Yeast surface display for protein engineering and characterization. Current Opinion in Structural Biology, 17(4): 467–473
Gietz R D, Schiestl R H (2007). High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nature Protocols, 2(1): 31–34
Glenn J K, Morgan M A, Mayfield M B, Kuwahara M, Gold M H (1983). An extracellular H2O2-requiring enzyme preparation involved in lignin biodegradation by the white rot basidiomycete Phanerochaete chrysosporium. Biochemical and Biophysical Research Communications, 114(3): 1077–1083
Guo J, Liu X, Zhang X, Wu J, Chai C, Ma D, Chen Q, Xiang D, Ge W (2019). Immobilized lignin peroxidase on Fe3O4@SiO2@polydopa-mine nanoparticles for degradation of organic pollutants. International Journal of Biological Macromolecules, 138: 433–440
Ilamathi R, Merline Sheela A, Nagendra Gandhi N (2019). Comparative evaluation of Pseudomonas species in single chamber microbial fuel cell with manganese coated cathode for reactive azo dye removal. International Biodeterioration & Biodegradation, 144: 104744
Jarosz-Wilkolazka A, Kochmanska Rdest J, Malarczyk E, Wardas W, Leonowicz A (2002). Fungi and their ability to decolorize azo and anthraquinonic dyes. Enzyme and Microbial Technology, 30(4): 566–572
Kabe Y, Osawa T, Ishihara A, Kabe T (2005). Decolorization of coal humic acid by extracellular enzymes produced by white-rot fungi. Coal Preparation, 25(4): 211–220
Khindaria A, Yamazaki I, Aust S D (1996). Stabilization of the veratryl alcohol cation radical by lignin peroxidase. Biochemistry, 35(20): 6418–6424
Kirk T K, Farrell R L (1987). Enzymatic “combustion”: The microbial degradation of lignin. Annual Review of Microbiology, 41(1): 465–501
Martins M A, Ferreira I C, Santos I M, Queiroz M J, Lima N (2001). Biodegradation of bio accessible textile azo dyes by Phanerochaete chrysosporium. Journal of Biotechnology, 89(2–3): 91–98
Nam S, Renganathan V (2000). Non-enzymatic reduction of azo dyes by NADH. Chemosphere, 40(4): 351–357
Pérez-Boada M, Ruiz-Dueñas F J, Pogni R, Basosi R, Choinowski T, Martínez M J, Piontek K, Martínez AT (2005). Versatile peroxidase oxidation of high redox potential aromatic compounds: site-directed mutagenesis, spectroscopic and crystallographic investigations of three long-range electron transfer pathways. Journal of Molecular Biology, 354(2): 385–402
Ribeiro G F, Côrte-Real M, Johansson B (2006). Characterization of DNA damage in yeast apoptosis induced by hydrogen peroxide, acetic acid, and hyperosmotic shock. Molecular Biology of the Cell, 17(10): 4584–4591
Romero J O, Fernández-Fueyo E, Avila-Salas F, Recabarren R, Alzate-Morales J, Martínez AT (2019). Binding and catalytic mechanism of veratryl alcohol oxidation by lignin peroxidase: A theoretical and experimental study. Computational and Structural Biotechnology Journal, 17: 1066–1074
Ruiz-Dueñas F J, Morales M, Mate M J, Romero A, Martínez M J, Smith A T, Martínez Á T (2008). Site-directed mutagenesis of the catalytic tryptophan environment in Pleurotus eryngii versatile peroxidase. Biochemistry, 47(6): 1685–1695
Ryu K, Hwang S Y, Kim K H, Kang J H, Lee E K (2008). Functionality improvement of fungal lignin peroxidase by DNA shuffling for 2,4-dichlorophenol degradability and H2O2 stability. Journal of Biotechnology, 133(1): 110–115
Saratale R G, Saratale G D, Chang J S, Govindwar S P (2011). Bacterial decolorization and degradation of azo dyes: A review. Journal of Taiwan Institute of Chemical Engineers, 42(1): 138–157
Singh M V, Anthony Weil P (2002). A method for plasmid purification directly form yeast. Analytical Biochemistry, 307(1): 13–17
Smith A T, Doyle W A, Dorlet P, Ivancich A (2009). Spectroscopic evidence for an engineered, catalytically active Trp radical that creates the unique reactivity of lignin peroxidase. Proceedings of the National Academy of Sciences of the United States of America, 106 (38): 16084–16089
Smith A T, Veitch N C (1998). Substrate binding and catalysis in hem peroxidases. Current Opinion in Chemical Biology, 2(2): 269–278
Stolz A (2001). Basic and applied aspects in the microbial degradation of azo dyes. Applied Microbiology and Biotechnology, 56(1–2): 69–80
Tinoco R, Verdín J, Vázquez-Duhalt R (2007). Role of oxidizing mediators and tryptophan 172 in the decoloration of industrial dyes by the versatile peroxidase from Bjerkandera adusta. Journal of Molecular Catalysis. B, Enzymatic, 46(1–4): 1–7
Ventura-Camargo B C, Marin-Morales M A (2013). Azo dyes: characterization and toxicity—A review. TLIST, 2(2): 85–103
Wesenberg D, Kyriakides I, Agathos S N (2003). White- rot fungi and their enzymes for the treatment of industrial dye effluents. Biotechnology Advances, 22(1–2): 161–187
Zhang Y, Geißen S U (2010). In vitro degradation of carbamazepine and diclofenac by crude lignin-peroxidase. Journal of Hazardous Materials, 176(1–3): 1089–1092
Acknowledgements
We thank Professor Dane Wittrup, Massachusetts Institute of Technology, for providing the pCTCON2 vector and S. cerevisiae EBY100 competent cells, and Dr. Helga Schinkel, Fraunhofer IME (Aachen, Germany) for valuable discussions. This work was supported by funds from the Ministry of Education, Science and Technological Development of the Republic of Serbia via project numbers ON172049, ON173017 and III46010.
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• Mutations in Lignin peroxidase Trp171 environment improved azo dyes degradation.
• Expression on yeast cell surface and cell lysis allowed reusability of biocatalyst.
• Aga2-LiP chimeric variants were characterized.
Supporting Information
Rights and permissions
About this article
Cite this article
Ilić Đurđić, K., Ostafe, R., Prodanović, O. et al. Improved degradation of azo dyes by lignin peroxidase following mutagenesis at two sites near the catalytic pocket and the application of peroxidase-coated yeast cell walls. Front. Environ. Sci. Eng. 15, 19 (2021). https://doi.org/10.1007/s11783-020-1311-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11783-020-1311-4