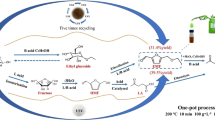
Abstract
With the aim of achieving a high 5-hydroxymethylfurfural (HMF) yield from glucose with H-ZSM-5 catalyst at low cost, three inexpensive biphasic reaction systems, H2O—tetrahydrofuran (THF), H2O—2-methyltetrahydrofuran (MeTHF) and H2O’2-butanol, were discovered and proved to be particularly effective in promoting the formation of HMF from glucose over H-ZSM-5 zeolite. In order to determine the optimal process conditions, the effects of various experimental variables, such as reaction temperature, reaction time, catalyst dosage, volume of organic solvent, as well as inorganic salt type on glucose conversion to HMF in three systems were investigated in detail. It was found that under optimal reaction conditions, H2O—THF, H2O—2-butanol and H2O—MeTHF allowed the glucose dehydration process to achieve HMF yields of up to 61%, 59%, and 50%, respectively. Moreover, in the three biphasic systems, the H-ZSM-5 catalyst was also demonstrated to maintain excellent stability. Thus, the catalytic approach proposed in this paper can be believed to have potential prospects for industrially efficient and low-cost production of HMF.
摘要
在本文中, H2O−四氢呋喃、H2O—2-丁醇、H2O−2-甲基四氢呋喃三种廉价的双相反应体系被发现并证实可以高效地促进H-ZSM-5 分子筛催化葡萄糖转化制备5-羟甲基糠醛(HMF). 为了在三种反应体系中获得最佳的HMF 产率, 研究了包括反应温度、反应时间、催化剂用量、有机相体积以及无机盐种类在内的不同反应参数对HMF 产率的影响. 研究发现, 最佳反应条件下, H-ZSM-5 分子筛催化葡萄糖转化制备HMF 在H2O—四氢呋喃、H2O—2-丁醇、H2O−2-甲基四氢呋喃三种体系中分别可获得高达61%、59%以及50%的产率. 这些结果证实上述三种高效的反应体系可以实现对昂贵的离子液体反应体系进行替代. 而且更重要的是, 在上述三种反应体系中, H-ZSM-5 分子筛在经历多次循环使用后依然可以保持催化活性和物质结构的稳定性. 因此我们相信, 本文中所呈现的高效、廉价的催化体系在HMF 的实际生产过程中将会具有极大的应用前景.
Similar content being viewed by others
References
van NGUYEN C, LIAO Y T, KANG T C. A metal-free, high nitrogen-doped nanoporous graphitic carbon catalyst for an effective aerobic HMF-to-FDCA conversion [J]. Green Chemistry, 2016, 18(22): 5957–5961. DOI: https://doi.org/10.1039/C6GC02118B.
OSATIASHTIANI A, LEE A F, GRANOLLERS M. Hydrothermally stable, conformal, sulfated zirconia monolayer catalysts for glucose conversion to 5-HMF [J]. ACS Catalysis, 2015, 5(7): 4345–4352. DOI: https://doi.org/10.1021/acscatal.5b00965.
TONG X, MA Y, LI Y. Biomass into chemicals: Conversion of sugars to furan derivatives by catalytic processes [J]. Applied Catalysis A: General, 2010, 385 (1, 2)}: 1–13. DOI: https://doi.org/10.1016/j.apcata.2010.06.049.
XU S, YAN X, BU Q. Highly efficient conversion of carbohydrates into 5-hydroxymethylfurfural using the bi-functional CrPO4 catalyst[J]. RSC Advances, 2016, 6(10): 8048–8052. DOI: https://doi.org/10.1039/C5RA23716E.
BAO Q, QIAO K, TOMIDA D. Preparation of 5-hydroymethylfurfural by dehydration of fructose in the presence of acidic ionic liquid [J]. Catalysis Communications, 2008, 9(6): 1383–1388. DOI: https://doi.org/10.1016/j.catcom.2007.12.002.
ROSATELLA A A, SIMEONOV S P, FRADE R F M. 5-hydroxymethylfurfural (HMF) as a building block platform: Biological properties, synthesis and synthetic applications [J]. Green Chemistry, 2011, 13(4): 754–793. DOI: https://doi.org/10.1039/C0GC00401D.
YONG G, ZHANG Y, YING J Y. Efficient catalytic system for the selective production of 5-hydroxymethylfurfural from glucose and fructose [J]. Angewandte Chemie International Edition, 2008, 47(48): 9345–9348. DOI: https://doi.org/10.1002/anie.200803207.
CHOUDHARY V, MUSHRIF S H, HO C. Insights into the interplay of Lewis and Brønsted acid catalysts in glucose and fructose conversion to 5-(hydroxymethyl) furfural and levulinic acid in aqueous media [J]. Journal of the American Chemical Society, 2013, 135(10): 3997–4006. DOI: https://doi.org/10.1021/ja3122763.
MORENO-RECIO M, SANTAMARÍA-GONZÁLEZ J, MAIRELES-TORRES P. Brönsted and Lewis acid ZSM-5 zeolites for the catalytic dehydration of glucose into 5-hydroxymethylfurfural [J]. Chemical Engineering Journal, 2016, 303: 22–30. DOI: https://doi.org/10.1016/j.cej.2016.05.120.
QI X, WATANABE M, AIDA T M. Catalytic conversion of cellulose into 5-hydroxymethylfurfural in high yields via a two-step process [J]. Cellulose, 2011, 18(5): 1327–1333. DOI: https://doi.org/10.1007/s10570-011-9568-1.
MO H, CHEN X, LIAO X. Sustainable synthesis of 5-hydroxymethylfurfural from waste cotton stalk catalyzed by solid superacid-SO4 2−/ZrO2 [J]. Journal of Central South University, 2017, 24(8): 1745–1753. DOI: https://doi.org/10.1007/s11771-017-3582-x.
XU S, PAN D, WU Y. Catalytic conversion of xylose and xylan into furfural over Cr3+/P-SBA-15 catalyst derived from spent adsorbent [J]. Industrial & Engineering Chemistry Research, 2019, 58(29): 13013–13020. DOI: https://doi.org/10.1021/acs.iecr.9b01821.
XU S, PAN D, WU Y. Direct conversion of wheat straw components into furan compounds using a highly efficient and reusable SnCl2-PTA/β zeolite catalyst [J]. Industrial & Engineering Chemistry Research, 2019, 58(22): 9276–9285. DOI: https://doi.org/10.1021/acs.iecr.9b00984.
FAN C, GUAN H, ZHANG H. Conversion of fructose and glucose into 5-hydroxymethylfurfural catalyzed by a solid heteropolyacid salt [J]. Biomass & Bioenergy, 2011, 35(7): 2659–2665. DOI: https://doi.org/10.1016/j.biombioe.2011.03.004.
OLSON D H, KOKOTAILO G T, LAWTON S L. Crystal structure and structure-related properties of ZSM-5 [J]. The Journal of Physical Chemistry, 1981, 85(15): 2238–2243. DOI: https://doi.org/10.1021/j150615a020.
SHIRAZI L, JAMSHIDI E, GHASEMI M R. The effect of Si/Al ratio of ZSM-5 zeolite on its morphology, acidity and crystal size [J]. Crystal Research and Technology: Journal of Experimental and Industrial Crystallography, 2008, 43(12): 1300–1306. DOI: https://doi.org/10.1002/crat.200800149.
JADHAV H, TAARNING E, PEDERSEN C M. Conversion of D-glucose into 5-hydroxymethylfurfural (HMF) using zeolite in [Bmim]Cl or tetrabutylammonium chloride (TBAC)/CrCl2 [J]. Tetrahedron Letters, 2012, 53(8): 983–985. DOI: https://doi.org/10.1016/j.tetlet.2011.12.059.
RAMLI N A S, AMIN N A S. Fe/HY zeolite as an effective catalyst for levulinic acid production from glucose: Characterization and catalytic performance [J]. Applied Catalysis B: Environmental, 2015, 163: 487–498. DOI: https://doi.org/10.1016/j.apcatb.2014.08.031.
LONG R Q, YANG R T. Reaction mechanism of selective catalytic reduction of NO with NH3 over Fe-ZSM-5 catalyst [J]. Journal of Catalysis, 2002, 207(2): 224–231. DOI: https://doi.org/10.1006/jcat.2002.3528.
PAGAN-TORRES Y J, WANG T, GALLO J M R. Production of 5-hydroxymethylfurfural from glucose using a combination of Lewis and Brønsted acid catalysts in water in a biphasic reactor with an alkylphenol solvent [J]. ACS Catalysis, 2012, 2(6): 930–934. DOI: https://doi.org/10.1021/cs300192z.
XIA H, HU H, XU S. Catalytic conversion of glucose to 5-hydroxymethyfural over Fe/β zeolites with extra-framework isolated Fe species in a biphasic reaction system [J]. Biomass & Bioenergy, 2018, 108: 426–432. DOI: https://doi.org/10.1016/j.biombioe.2017.12.007.
LI H, DENG A, REN J. A modified biphasic system for the dehydration of D-xylose into furfural using SO4 2−/TiO2-ZrO2/La3+ as a solid catalyst [J]. Catalysis Today, 2014, 234: 251–256. DOI: https://doi.org/10.1016/j.cattod.2013.12.043.
MORAIS A R C, BOGEL-LUKASIK R. Highly efficient and selective CO2-adjunctive dehydration of xylose to furfural in aqueous media with THF[J]. Green Chemistry, 2016, 18(8): 2331–2334. DOI: https://doi.org/10.1039/C5GC02863A.
COMBS E, CINLAR B, PAGAN-TORRES Y, DUMESIC J A, SHANKS B H. Influence of alkali and alkaline earth metal salts on glucose conversion to 5-hydroxymethylfurfural in an aqueous system [J]. Catalysis Communications, 2013, 30: 1–4. DOI: https://doi.org/10.1016/j.catcom.2012.10.011.
TSILOMELEKIS G, ORELLA M J, LIN Z. Molecular structure, morphology and growth mechanisms and rates of 5-hydroxymethyl furfural (HMF) derived humins [J]. Green Chemistry, 2016, 18(7): 1983–1993. DOI: https://doi.org/10.1039/C5GC01938A.
PATIL S K R, HELTZEL J, LUND C R F. Comparison of structural features of humins formed catalytically from glucose, fructose, and 5-hydroxymethylfurfuraldehyde [J]. Energy & Fuels, 2012, 26(8): 5281–5293. DOI: https://doi.org/10.1021/ef3007454.
JIMÉNEZ-MORALES I, MORENO-RECIO M, SANTAMARÍA-GONZÁLEZ J. Production of 5-hydroxymethylfurfural from glucose using aluminium doped MCM-41 silica as acid catalyst [J]. Applied Catalysis B: Environmental, 2015, 164: 70–76. DOI: https://doi.org/10.1016/j.apcatb.2014.09.002.
HU S, ZHANG Z, ZHOU Y. Conversion of fructose to 5-hydroxymethylfurfural using ionic liquids prepared from renewable materials [J]. Green Chemistry, 2008, 10(12): 1280–1283. DOI: https://doi.org/10.1039/B810392E.
OTOMO R, YOKOI T, KONDO J N. Dealuminated Beta zeolite as effective bifunctional catalyst for direct transformation of glucose to 5-hydroxymethylfurfural [J]. Applied Catalysis A: General, 2014, 470: 318–326. DOI: https://doi.org/10.1016/j.apcata.2013.11.012.
OTOMO R, TATSUMI T, YOKOI T. Beta zeolite: A universally applicable catalyst for the conversion of various types of saccharides into furfurals [J]. Catalysis Science & Technology, 2015, 5(8): 4001–4007. DOI: https://doi.org/10.1039/C5CY00719D.
FOGER K, SANDERS J V, SEDDON D. Channel arrangements and activity of some ZSM zeolites [J]. Zeolites, 1984, 4(4): 337–345. DOI: https://doi.org/10.1016/0144-2449(84)90009-5.
SWIFT T D, NGUYEN H, ERDMAN Z. Tandem Lewis acid/Br0nsted acid-catalyzed conversion of carbohydrates to 5-hydroxymethylfurfural using zeolite beta [J]. Journal of Catalysis, 2016, 333: 149–161. DOI: https://doi.org/10.1016/j.jcat.2015.10.009.
GIRISUTA B, JANSSEN L, HEERES H J. A kinetic study on the decomposition of 5-hydroxymethylfurfural into levulinic acid [J]. Green Chemistry, 2006, 8(8): 701–709. DOI: https://doi.org/10.1039/b518176c.
PENG L, LIN L, ZHANG J. Catalytic conversion of cellulose to levulinic acid by metal chlorides [J]. Molecules, 2010, 15(8): 5258–5272. DOI: https://doi.org/10.3390/molecules15085258.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Project(3207049713) supported by the Scientific Research Foundation of Graduate School of Southeast University, China
Rights and permissions
About this article
Cite this article
Xu, Sq., Pan, Dh. & Xiao, Gm. Enhanced HMF yield from glucose with H-ZSM-5 catalyst in water-tetrahydrofuran/2-butanol/2-methyltetrahydrofuran biphasic systems. J. Cent. South Univ. 26, 2974–2986 (2019). https://doi.org/10.1007/s11771-019-4229-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-019-4229-x