Abstract
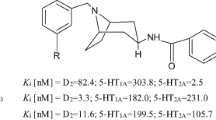
Eptapirone free base, F11440,4-methyl-2-(4-(4-(pyrimidin-2-yl)piperazin-1-yl)butyl)-1,2,4-triazine-3,5(2H,4H)-dione, represents a potent and selective 5-HT1A receptor agonist with high efficacy and the potential to regulate anxiety disorders. Herein, we report a method to retro-synthesize eptapirone free base. The compound consists of heterocyclic aromatic portion and aliphatic portion, and the synthetic route consisted of a total of nine steps with an overall yield of 8.8% starting from the commercially available materials. The key steps in the synthetic method involved: (1) using sodium hydroxide and ethylene glycol as solvent resulted in a better cyclization and yield (61.6%) of 1,2,4-triazine-3,5(2H,4H)-dione; (2) an acceptable yield (63.1%) of 4-tert-butyl(pyrimidin-2-yl)piperazine-1-carboxylate was obtained under an optimized conditions of using triethylamine as a base, ethanol as a solvent, and a reaction temperature of 50 °C for 16 h with non-metal catalysis and less byproducts; (3) the reaction step of eptapirone could get a better yield (49.6%) with an optimized condition of potassium carbonate as a base, acetonitrile as a solvent, NaI as a catalyst, and a reaction temperature of 50 °C for 12 h by nucleophilic substitution reaction. The main advantages of this route were an acceptable product purity, the commercial availability of all starting materials and the absence of high temperature, high pressure and noble metal catalysts, which could result in more feasible commercial applications.












Similar content being viewed by others
References
Cadieux RJ (1996) Azapirones: an alternative to benzodiazepines for anxiety. Am Fam Physician 53:2349–2353
Carvalho AF, Machado JR, Cavalcante JL (2009) Augmentation strategies for treatment-resistant depression. Curr Opin Psychiatry 22:7–12. https://doi.org/10.1097/YCO.0b013e32831be9ef
Celada P, Bortolozzi A, Artigas F (2013) Serotonin 5-HT1A receptors as targets for agents to treat psychiatric; disorders: rationale and current status of research. CNS Drugs 27:703–716. https://doi.org/10.1007/s40263-013-0071-0
Chang PK, Ulbricht TLV (1958) The Reaction of chloral hydrate with semicarbazides and the synthesis of semicarbazide-C14 and 6-Azauracil-2-C14. J Am Chem Soc 80:976–979
Deutsch DH et al (1975) Cyclization of glyoxylic acid semicarbazone. US 3922273A
Duncton MAJ, Roffey JRA, Hamlyn RJ, Adams DR (2006) Parallel synthesis of N-arylpiperazines using polymer-assisted reactions. Tetrahedron Lett 47:2549–2552. https://doi.org/10.1016/j.tetlet.2006.02.047
Gustavsson A, Svensson M, Jacobi F, Allgulander C, Alonso J, Beghi E et al (2011) Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol 21:718–779. https://doi.org/10.1016/j.euroneuro.2011.08.008
Kishi T, Meltzer HY, Iwata N et al (2013) Augmentation of antipsychotic drug action by azapirone 5-HT1A receptor partial agonists: a meta-analysis. Int J Neuropsychopharmacol 16:1259–1266. https://doi.org/10.1017/S1461145713000151
Koek W, Patoiseau JF, Assié MB, Cosi C, Kleven MS, Dupontpasselaigue E et al (1998) F 11440, a potent, selective, high efficacy 5-HT1A receptor agonist with marked anxiolytic and antidepressant potential. J Pharmacol Exp Ther 287:266–283
Kumar JSD, Prabhakaran J, Majo VJ, Milak M et al (2007) Synthesis and in vivo evaluation of a novel 5-HT 1A receptor agonist radioligand [O-methyl-11 C]2-(4-(4-(2-methoxyphenyl) piperazin-1-yl)butyl)-4-methyl-1,2,4-triazine-3,5(2H,4H)dione in nonhuman primates. Eur J Nucl Med Mol Imaging 34:1050–1060. https://doi.org/10.1007/s00259-006-0324-y
Kumar JSD, Majo VJ, Prabhakaran J, Mann JJ et al (2014) Synthesis and evaluation of arylpiperazines derivatives of 3,5-dioxo-(2H,4H)-1,2,4-triazine as 5-HT1AR ligands. Bioorg Med Chem Lett 24:4759–4762. https://doi.org/10.1016/j.bmcl.2014.07.048
Majo VJ, Milak MS, Prabhakaran J, Mali P, Savenkova L, Simpson NR et al (2013) Synthesis and in vivo evaluation of [(18)F]2-(4-(4-(2-(2-fluoroethoxy)phenyl)piperazin-1-yl)butyl)-4-methyl-1,2,4-tri azine-3,5(2H,4H)-dione ([(18)F]FECUMI-101) as an imaging probe for 5-HT1A receptor agonist in nonhuman primates. Bioorg Med Chem 21:5598–5604. https://doi.org/10.1016/j.bmc.2013.05.050
Maslen HL, Hughes D, Hursthouse M, Clercq ED, Balzarini J, Simons C (2004) 6-Azapyrimidine-2’-deoxy-4’-thionucleosides: antiviral Agents against TK+ and TK− HSV and VZV Strains. J Med Chem 47:5482–5491. https://doi.org/10.1021/jm049806q
Mohammadi A, Eshghi H, Bakavoli M, Hadizadeh F, Moradi H (2016) Synthesis of novel 3-substituted-5H-benzo[5,6][1, 4]thiazino[3,2-e][1,2,4]triazines and their 15-lipoxygenase inhibitory activity. J Iran Chem Soc 13:1539–1547. https://doi.org/10.1007/s13738-016-0870-6
Murray CJL, Lopez AD et al (1997) Alternative projections of mortality and disability by cause 1990–2020: global Burden of Disease Study. Lancet 349:1498–1504. https://doi.org/10.1016/S0140-6736(96)07492-2
Newington IM, Wynn DG, Nairne RJD et al (2011) Piperazine derivatives and radiolabeled analogs as 5HT1A ligands and their preparation and use as imaging agent and in treatment of 5HT1A-associated diseases: WO Patent 2011150183
Perrone R, Berardi F, Colabufo NA et al (2001) trans-4-[4-(Methoxyphenyl)cyclohexyl]-1-arylpiperazines: a new class of potent and selective 5-HT(1A) receptor ligands as conformationally constrained analogues of 4-[3-(5-methoxy-1,2,3,4-tetrahydronaphthalen-1-yl)propyl]-1-arylpiperazines. J Med Chem 44:4431–4442. https://doi.org/10.1021/jm010866v
Prabhakaran J, Parsey RV, Majo VJ, Hsiung SC et al (2006) Synthesis, in vitro and in vivo evaluation of [O-methyl-11C]2-{4-[4-(3-methoxyphenyl)piperazin-1-yl]-butyl}-4-methyl-2H-[1,2,4]-triazine-3,5-dione: a novel agonist 5-HT 1A receptor PET ligand. Bioorg Med Chem Lett 16:2101–2104. https://doi.org/10.1016/j.bmcl.2006.01.052
Prinssen EPM, Colpaert FC, Koek W (2002) 5-HT1A receptor activation and anti-cataleptic effects: high-efficacy agonists maximally inhibit haloperidol-induced catalepsy. Eur J Pharmacol 453:217–221. https://doi.org/10.1016/S0014-2999(02)02430-5
Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D et al (2006) Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163:1905–1917. https://doi.org/10.1176/ajp.2006.163.11.1905
Salituro FG, Saunders JO et al (2011) Preparation of piperazinylcarbonyl benzenesulfonamides for use in the treatment of cancer characterized as having an IDH mutation WO2011072174
Taylor DP, Stephenson DT et al (2004) Method of using a cox-2 inhibitor and a 5-HT1A receptor modulator as a combination therapy: US Patent 2004/0147581 A1. 2004-7-29
Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L et al (2006) Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 163:28–40. https://doi.org/10.1176/appi.ajp.163.1.28
Üstün TB, Ayusomateos JL, Chatterji S, Mathers C, Murray CJL (2004) Global burden of depressive disorders in the year 2000. Br J Psychiatry 184:386–392
Wang BL, Shi YX, Ma Y, Liu XH, Li YH, Song HB et al (2010) Synthesis and biological activity of some novel trifluoromethyl-substituted 1,2,4-triazole and bis(1,2,4-triazole) Mannich bases containing piperazine rings. J Agric Food Chem 58:5515–5522. https://doi.org/10.1021/jf100300a
Acknowledgements
This work was financially supported by the National Science Foundation of China (No. 21576295).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Peng, W., Chen, J., Liu, H. et al. An improved synthesis of the 5-HT1A receptor agonist Eptapirone free base. Chem. Pap. 73, 1321–1331 (2019). https://doi.org/10.1007/s11696-019-00685-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-019-00685-1