Abstract
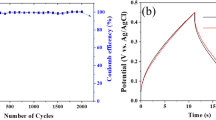
Graphene oxide (GO) was prepared by a modified Hummers method. TiO2 nanoparticles (TiO2 NPs) and NaOH were added into the GO suspension to prepare TiO2 nanowires-reduced GO (TiO2 NWs-rGO) nanocomposite by one-step hydrothermal synthesis. Effects of the initial mass ratio of TiO2 NPs and GO on the morphologies and electrochemical properties of the nanocomposite were investigated in detail. The results show that TiO2 NWs with the length of ~ 10 μm and the diameter of ~ 50 nm are homogeneously distributed on the graphene nanosheets when the initial mass ratio of TiO2 NPs and GO is 1:4. Its specific capacitance of the TiO2 NWs-rGO nanocomposite can reach 572 F·g−1 at 1 A·g−1 in 6 M KOH. Moreover, the nanocomposite exhibits a long-term cycle stability, ~ 84% specific capacitance after 5000 cycles at 10 A·g−1. It suggests that it has potential as an electrode material for high-performance electrochemical supercapacitors.







Similar content being viewed by others
References
Inagaki M, Konno H, Tanaike O (2010) Carbon materials for electrochemical capacitors. J Power Sources 195(24):7880–7903
Zhang LL, Zhao XS (2009) Carbon-based materials as supercapacitor electrodes. Chem Soc Rev 38(9):2520–2531
Liu C, Yu Z, Neff D, Zhamu A, Jang BZ (2010) Graphene-based supercapacitor with an ultrahigh energy density. Nano Lett 10(12):4863–4868
Beguin F, Presser V, Balducci A, Frackowiak E (2014) Carbons and electrolytes for advanced supercapacitors. Adv Mater 26(14):2219–2251 2283
Wang H, Yi H, Chen X, Wang X (2014) One-step strategy to three-dimensional graphene/VO2 nanobelt composite hydrogels for high performance supercapacitors. J Mater Chem A 2(4):1165–1173
Dong X, Wang X, Wang J, Song H, Li X, Wang L, Chan-Park MB, Li CM, Chen P (2012) Synthesis of a MnO2–graphene foam hybrid with controlled MnO2 particle shape and its use as a supercapacitor electrode. Carbon 50(13):4865–4870
Kim D, Yun J, Lee G, Ha JS (2014) Fabrication of high performance flexible micro-supercapacitor arrays with hybrid electrodes of MWNT/V2O5 nanowires integrated with a SnO2 nanowire UV sensor. Nanoscale 6(20):12034–12041
Foo CY, Sumboja A, Tan DJH, Wang J, Lee PS (2014) Flexible and highly scalable V2O5-rGO electrodes in an organic electrolyte for supercapacitor devices. Adv Energy Mater 4(12):3412–3420
Qian T, Xu N, Zhou J, Yang T, Liu X, Shen X, Liang J, Yan C (2015) Interconnected three-dimensional V2O5/polypyrrole network nanostructures for high performance solid-state supercapacitors. J Mater Chem A 3(2):488–493
Hu CC, Hsu TC, Kao LH (2012) One-step cohydrothermal synthesis of nitrogen-doped titanium oxide nanotubes with enhanced visible light photocatalytic activity. Int J Photoenergy 2012:1–9
Das M, Datta J, Dey A, Jana R, Layek A, Middya S, Ray PP (2015) One step hydrothermal synthesis of a rGO–TiO2 nanocomposite and its application on a Schottky diode: improvement in device performance and transport properties. RSC Adv 5(123):101582–101592
Yang S, Lin Y, Song X, Zhang P, Gao L (2015) Covalently coupled ultrafine H-TiO2 nanocrystals/nitrogen-doped graphene hybrid materials for high-performance supercapacitor. ACS Appl Mat Interfaces 7(32):17884–17892
Al-Rubaye S, Rajagopalan R, Subramaniyam CM, Yu Z, Dou SX, Cheng Z (2016) Electrochemical performance enhancement in MnCo2O4 nanoflake/graphene nanoplatelets composite. J Power Sources 324:179–187
Zhai T, Wan L, Sun S, Chen Q, Sun J, Xia Q, Xia H (2017) Phosphate ion functionalized Co3O4 ultrathin nanosheets with greatly improved surface reactivity for high performance pseudocapacitors. Adv Mater 29(7):1604167
He X, Yang CP, Zhang GL, Shi DW, Huang QA, Xiao HB, Liu Y, Xiong R (2016) Supercapacitor of TiO2 nanofibers by electrospinning and KOH treatment. Mater Des 106:74–80
Ding Y, Bai W, Sun J, Wu Y, Memon MA, Wang C, Liu C, Huang Y, Geng J (2016) Cellulose tailored anatase TiO2 nanospindles in three-dimensional graphene composites for high-performance supercapacitors. ACS Appl Mat Interfaces 8(19):12165–12175
Liu Y, Gao T, Xiao H, Guo W, Sun B, Pei M, Zhou G (2017) One-pot synthesis of rice-like TiO2/graphene hydrogels as advanced electrodes for supercapacitors and the resulting aerogels as high-efficiency dye adsorbents. Electrochim Acta 229:239–252
Weiss NO, Zhou H, Liao L, Liu Y, Jiang S, Huang Y, Duan X (2012) Graphene: an emerging electronic material. Adv Mater 24(43):5782–5825
Hassoun J, Bonaccorso F, Agostini M, Angelucci M, Betti MG, Cingolani R, Gemmi M, Mariani C, Panero S, Pellegrini V, Scrosati B (2014) An advanced lithium-ion battery based on a graphene anode and a lithium iron phosphate cathode. Nano Lett 14(8):4901–4906
Ke Q, Liao Y, Yao S, Song L, Xiong X (2015) A three-dimensional TiO2/graphene porous composite with nano-carbon deposition for supercapacitor. J Mater Sci 51(4):2008–2016
Zhou H, Zhang Y (2014) Electrochemically self-doped TiO2 nanotube arrays for supercapacitors. J Phys Chem C 118(11):5626–5636
Goriparti S, Miele E, Prato M, Scarpellini A, Marras S, Monaco S, Toma A, Messina GC, Alabastri A, De Angelis F, Manna L, Capiglia C, Zaccaria RP (2015) Direct synthesis of carbon-doped TiO2-bronze nanowires as anode materials for high performance lithium-ion batteries. ACS Appl Mat Interfaces 7(45):25139–25146
Ramadoss A, Kim G-S, Kim SJ (2013) Fabrication of reduced graphene oxide/TiO2 nanorod/reduced graphene oxide hybrid nanostructures as electrode materials for supercapacitor applications. CrystEngComm 15(47):10222–10229
Xiang C, Li M, Zhi M, Manivannan A, Wu N (2012) Reduced graphene oxide/titanium dioxide composites for supercapacitor electrodes: shape and coupling effects. J Mater Chem 22(36):19161–19167
Sun X, Xie M, Travis JJ, Wang G, Sun H, Lian J, George SM (2013) Pseudocapacitance of amorphous TiO2 thin films anchored to graphene and carbon nanotubes using atomic layer deposition. J Phys Chem C 117(44):22497–22508
Salari M, Aboutalebi SH, Chidembo AT, Nevirkovets IP, Konstantinov K, Liu HK (2012) Enhancement of the electrochemical capacitance of TiO2 nanotube arrays through controlled phase transformation of anatase to rutile. Phys Chem Chem Phys 14(14):4770–4779
An KH, Kim WS, Park YS, Choi YC, Lee SM, Chung DC (2010) Supercapacitors using singlewalled carbon nanotube electrodes. Adv Mater 13(7):497–500
Bavykin DV, Parmon VN, Lapkin AA, Walsh FC (2004) The effect of hydrothermal conditions on the mesoporous structure of TiO2 nanotubes. J Mater Chem 14(22):3370–3377
Huang J, Cao Y, Deng Z, Tong H (2011) Formation of titanate nanostructures under different NaOH concentration and their application in wastewater treatment. J Solid State Chem 184(3):712–719
Ramadoss A, Kim SJ (2013) Improved activity of a graphene–TiO2 hybrid electrode in an electrochemical supercapacitor. Carbon 63:434–445
Pan X, Zhao Y, Liu S, Korzeniewski CL, Wang S, Fan Z (2012) Comparing graphene-TiO2 nanowire and graphene-TiO2 nanoparticle composite photocatalysts. ACS Appl Mat Interfaces 4 (8):3944
Funding
This work is supported by the Natural Science Foundation of Heilongjiang Province (LC2015020), Technology Foundation for Selected Overseas Chinese Scholar, Ministry of Personnel of China (2015192), the Innovative Talent Fund of Harbin city (2016RAQXJ185), and Science Funds for the Young Innovative Talents of HUST (201604).
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• One-step hydrothermal synthesis of TiO2 nanowires-reduced graphene oxide composite (TiO2NWs-rGO).
• The initial mass ratio of TiO2 nanoparticles and GO has a great effect on the morphologies and electrochemical properties of the composite.
• The composite exhibits a specific capacitance of 572F·g−1 at 1 A·g−1 in 6 M KOH.
• The composite exhibits a long-term cycle stability, retaining 84% specific capacitance after 5000 cycles at 10 A·g−1.
Electronic supplementary material
ESM 1
(DOC 2679 kb)
Rights and permissions
About this article
Cite this article
Yue, H.Y., Guan, E.H., Gao, X. et al. One-step hydrothermal synthesis of TiO2 nanowires-reduced graphene oxide nanocomposite for supercapacitor. Ionics 25, 2411–2418 (2019). https://doi.org/10.1007/s11581-018-2678-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-018-2678-0