Abstract
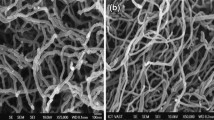
Lithium manganese phosphate (LiMnPO4) nanorods were synthesized using the modified polyol method. Polyvinylpyrrolidone was used as a stabilizer to control the shape and size of LiMnPO4 nanorods. Resin coating process was used to coat the carbon over the LiMnPO4 nanorods. X-ray diffraction and Fourier transform infrared spectroscopy results showed the formation of LiMnPO4 crystalline phase. The TEM image shows a uniform coating of the nano size (2.3 nm) carbon over the surface of LiMnPO4 nanorods and the EDS spectrum of the carbon-coated LiMnPO4 nanorods confirming the presence of carbon element along with the other Mn, P, and O elements. Impedance measurements were made on pure and carbon-coated LiMnPO4 nanorods, and their conductivities were evaluated by analyzing the measured impedance data using the WinFIT software. More than two orders of magnitude of conductivity enhancement was observed in the carbon-coated LiMnPO4 nanorods compared to pure ones, and the conductivity enhancement may be attributed to the presence of carbon over LiMnPO4 nanorods. Temperature dependence of conductivity and ac conductivity were calculated using impedance data of pure and carbon-coated LiMnPO4 nanorods. CR2032 type lithium ion coin cells were fabricated using pure and carbon-coated LiMnPO4 nanorods and characterized by measuring charge–discharge cycles between 2.9 and 4.5 V at room temperature. More than 25 % of improved capacity was achieved in the carbon-coated LiMnPO4 nanorods when compared to pure ones synthesized using modified polyol and resin coating processes.













Similar content being viewed by others
References
Nazri A, Pistoia G (2004) Lithium batteries: science and technology. Kluwer, Boston
Scrosati B (1995) Nature 373:557
Broussely M (1999) J Power Sources 81–82:140
Bates JB, Dudney NJ, Neudecker B, Ueda A, Evans CD (2000) Solid State Ion 135:33
Baudry P, Lascaud S, Majastre H, Bloch D (1997) J Power Sources 68:432
Owens BB, Smyrl WH, Xu JJ (1999) J Power Source 81–82:150
Tarascon JM, Armand M (2001) Nature 414:359
Winter M, Brodd RJ (2004) Chem Rev 104:4245
Scrosati B (2000) Electrochim Acta 45:2461
Novak P, Muller K, Santhanam KSV, Haas O (1997) Chem Rev 97:207
Xiao J, Xu W, Wang D, Choi D, Wang W, Li X, Graff GL, Liu J, Zhang JG (2010) J Electrochem Soc 157:A1047
Mizushima K, Jones PC, Wiseman PJ, Goodenough JB (1980) Mater Res Bull 15:783
Julien C (2003) Solid State Ion 157:57
Kalyani P, Kalaiselvi N (2005) Sci Tech Adv Mater 6:689
Ammundsen B, Paulsen J (2001) Adv Mater 13:943
Yang X, Tang W, Liu Z, Makita Y, Ooi K (2002) J Mater Chem 12:489
Fey GTK, Huang DL (1999) Electrochim Acta 45:295
Thackeray M (2002) Nat Mater 1:81
Dahn JR, Fuller EW, Obrovac M, Von Sacken U (1994) Solid State Ion 69:265
Blyr A, Sigala C, Amatucci G, Guyomard D, Chabre Y, Tarascon JM (1998) J Electrochem Soc 145:194
Padhi AK, Nanjundaswamy KS, Masquelier C, Okada S, Goodenough JB (1997) J Electrochem Soc 144:1609
Kang B, Ceder G (2009) Nature 458:190
Malik R, Burch D, Bazant M, Ceder G (2010) Nano Lett 10:4123
Lee KT, Kan WH, Nazar LF (2009) J Am Chem Soc 131:6044
Ellis BL, Lee KT, Nazar LF (2010) Chem Mater 22:691
Martha SK, Grinblat J, Haik O, Zinigrad E, Drezen T, Miners JH, Exnar I, Kay A, Markovsky B, Aurbach D (2009) Angew Chem 48:8559
Sinha NN, Munichandraiah N (2010) J Electrochem Soc 157:A824
Choi D, Wang D, Bae IT, Xiao J, Nie Z, Wang W, Viswanathan VV, Lee YJ, Zhang G, Graff GL, Yang Z, Liu J (2010) Nano Lett 10:2799
Bakenov Z, Taniguchi I (2010) Electrochem Commun 12:75
Kang B, Ceder G (2010) J Electrochem Soc 157:A808
Martha SK, Markovsky B, Grinblat J, Gofer Y, Haik O, Zinigrad E, Aurbach D, Drezen T, Wang D, Deghenghi G, Exnar I (2009) J Electrochem Soc 156:A541
Choi D, Xiao J, Choi YJ, Hardy JS, Vijayakumar M, Liu J, Xu W, Wang W, Zhang JG, Graff GL, Yang Z (2011) Energy Environ Sci 4:4560
Xiao J, Chernova NA, Upreti S, Chen X, Li Z, Deng Z, Choi D, Xu W, Nie Z, Graff GL, Liu J, Whittingham MS, Zhang JG (2011) Phys Chem Chem Phys 13:18099
Yang J, Xu JJ (2004) Electrochem Solid State Lett 7:A515
Franger S, Cras FL, Bourbon C, Rouault H (2003) J Power Sources 119:252
Kim DH, Im JS, Kang JW, Kim EJ, Ahn HN, Kim J (2007) J Nanosci Nanotechnol 7:3949
Kim DH, Kim J (2006) Electrochem Solid State Lett 9:A439
Kim DH, Kim J (2007) J Phys Chem Solids 68:734
Liu H, Yang H, Li J (2010) Electrochim Acta 55:1626
Myung ST, Komaba S, Hirosaki N, Yashiro H, Kumagai N (2004) Electrochem Acta 49:4213
Belharouak I, Johnson C, Amine K (2005) Electrochem Commun 7:983
Park KS, Son JT, Chung HT, Kim SJ, Lee CH, Kim HQ (2003) Electrochem Commun 5:839
Huang YH, Park KS, Goodenough JB (2006) J Electrochem Soc 153:A2282
Kim DK, Muralidharan P, Lee HW, Ruffo R, Yang Y, Chan CK, Peng H, Huggins RA, Cui Y (2008) Nano Lett 8:3948
Chan CK, Zhang XF, Cui Y (2008) Nano Lett 8:307
Muralidharan P, Venkateswarlu M, Satyanarayana N (2004) Solid State Ion 166:27
Macedo PB, Moynihan CT, Bose R (1972) Phys Chem Glasses 13:171
Provenzano V, Boesch LP, Volterra V, Moynihan CT, Macedo PB (1972) J Am Ceram Soc 55:492
Moynihan CT, Boesch LP, Laberge NL (1973) Phys Chem Glasses 14:122
Hayri EA, Greenblatt M (1987) J Non-Cryst Solids 94:387
Joncher AK (1977) Nature 267:673
Funke K (1993) Prog Solid State Chem 22:111
Jain H, Mundy JN (1987) J Non-Cryst Solids 91:315
Qian H, Lu JQ (2010) J Nanosci Nanotechnol 10:5346
Cole KS, Cole RH (1941) J Chem Phys 9:341
Frohlich H (1958) Theory of dielectrics. Oxford University Press, London
Acknowledgments
NS gratefully acknowledges DST, CSIR, UGC, DRDO and AICTE, Govt. of India, for providing financial support in the form of research projects. The authors are also grateful for the financial support from the Natural Sciences and Engineering Research Council (NSERC), Canada for the Discovery grant individual to MM, The Canadian Bureau for International Education, on behalf of Foreign Affairs and International Trade, Canada (DFAIT) for providing Graduate Student Exchange Program (GSEP) fellowship to RP.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, P.R., Venkateswarlu, M., Misra, M. et al. Enhanced conductivity and electrical relaxation studies of carbon-coated LiMnPO4 nanorods. Ionics 19, 461–469 (2013). https://doi.org/10.1007/s11581-012-0778-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-012-0778-9