Abstract
Purpose
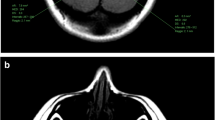
To perform T1 signal intensity (SI) measurements in the dentate nuclei of adult patients with confirmed multiple sclerosis (MS) after serial administrations of the macrocyclic gadolinium-based contrast agents (GBCAs), gadoterate meglumine and gadobutrol.
Materials and methods
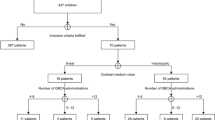
This retrospective study was approved by the institutional review board and informed consent was waived. A review of our PACS database for the period from March 1, 2007 to July 31, 2016 revealed 158 confirmed MS patients who received exclusively either gadoterate meglumine (n = 81) or gadobutrol (n = 77) for diagnosis and follow-up. SI measurements on unenhanced T1-weighted images were performed on all scans of all patients and at regions of interest (ROIs) positioned on the dentate nucleus (DN) and pons. The dentate nucleus-to-pons (DNP) T1-SI ratio was subsequently calculated. Unpaired T test and regression analysis were used to evaluate statistical differences.
Results
An increase in DNP was noted between the first and last MR examinations for both gadoterate meglumine (0.0032 ± 0.0216) and gadobutrol (0.0019 ± 0.0346). Although the differences were not statistically significant based across the entire patient population, visible T1 hyperintensity in the DN was noted in approximately one-third of all patients in each group that received at least five administrations of either GBCA.
Conclusions
SI increases on unenhanced T1-weighted images possibly indicative of gadolinium retention occur after serial administrations of the macrocyclic GBCAs, gadoterate meglumine and gadobutrol.





Similar content being viewed by others
References
Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D (2014) High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270:834-1
Errante Y, Cirimele V, Mallio CA, Di Lazzaro V, Zobel BB, Quattrocchi CC (2014) Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Invest Radiol 49:685–690
Quattrocchi CC, Mallio CA, Errante Y et al (2015) Gadodiamide and dentate nucleus T1 hyperintensity in patients with meningioma evaluated by multiple follow-up contrast enhanced magnetic resonance examinations with no systemic interval therapy. Invest Radiol 50:470–472
Quattrocchi CC, Mallio CA, Errante Y, Zobel BB (2015) High T1 signal intensity in dentate nucleus after multiple injections of linear gadolinium chelates. Radiology 276:616–617
Kanda T, Osawa M, Oba H et al (2015) High signal intensity in dentate nucleus on unenhanced T1-weighted MR images: association with linear versus macrocyclic gadolinium chelate administration. Radiology 275:803–809
Cao Y, Huang DQ, Shih G, Prince MR (2016) Signal change in the dentate nucleus on T1-weighted MR images after multiple administrations of gadopentetate dimeglumine versus gadobutrol. AJR Am J Roentgenol 206:414–419
Radbruch A, Weberling LD, Kieslich PJ et al (2016) Intraindividual analysis of signal intensity changes in the dentate nucleus after consecutive serial applications of linear and macrocyclic gadolinium-based contrast agents. Invest Radiol 51:683–690
Radbruch A, Weberling LD, Kieslich PJ et al (2015) High-signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted images: evaluation of the macrocyclic gadolinium-based contrast agent Gadobutrol. Invest Radiol 50:805–810
Radbruch A, Weberling LD, Kieslich PJ et al (2015) Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology 275:783–791
Weberling LD, Kieslich PJ, Kickingereder P et al (2015) Increased signal intensity in the dentate nucleus on unenhanced T1-weighted images after gadobenate dimeglumine administration. Invest Radiol 50:743–748
Ramalho J, Castillo M, AlObaidy M et al (2015) High signal intensity in globus pallidus and dentate nucleus on unenhanced T1-weighted MR images: evaluation of two linear gadolinium-based contrast agents. Radiology 276:836–844
Ramalho J, Semelka RC, AlObaidy M, Ramalho M, Nunes RH, Castillo M (2016) Signal intensity change on unenhanced T1-weighted images in dentate nucleus following gadobenate dimeglumine in patients with and without previous multiple administrations of gadodiamide. Eur Radiol 26:4080–4088
Roberts DR, Holden KR (2015) Progressive increase of T1 signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images in the pediatric brain exposed to multiple doses of gadolinium contrast. Brain Dev 38:331–336
Roberts DR, Chatterjee AR, Yazdani M et al (2016) Pediatric patients demonstrate progressive T1-weighted hyperintensity in the dentate nucleus following multiple doses of gadolinium-based contrast agent. AJNR Am J Neuroradiol 37:2340–2347
Flood TF, Stence NV, Maloney JA, Mirsky DM (2017) Pediatric brain: repeated exposure to Linear gadolinium-based contrast material is associated with increased signal intensity at unenhanced T1-weighted MR Imaging. Radiology 282:222–228
Hu HH, Pokorney A, Towbin RB, Miller JH (2016) Increased signal intensities in the dentate nucleus and globus pallidus on unenhanced T1-weighted images: evidence in children undergoing multiple gadolinium MRI exams. Pediatr Radiol 46:1590–1598
Stojanov DA, Aracki-Trenkic A, Vojinovic S, Benedeto-Stojanov D, Ljubisavljevic S (2016) Increasing signal intensity within the dentate nucleus and globus pallidus on unenhanced T1 W magnetic resonance images in patients with relapsing-remitting multiple sclerosis: correlation with cumulative dose of a macrocyclic gadolinium-based contrast agent, gadobutrol. Eur Radiol 26:807–815
Rossi Espagnet MC, Bernardi B, Pasquini L, Figà-Talamanca L, Tomà P, Napolitano A (2017) Signal intensity at unenhanced T1-weighted magnetic resonance in the globus pallidus and dentate nucleus after serial administrations of a macrocyclic gadolinium-based contrast agent in children. Pediatr Radiol. 2017 May 19. [Epub ahead of print]. Erratum in: Pediatr Radiol
Maeda H, Sato M, Yoshikawa A, Kimura M, Sonomura T, Terada M, Kishi K (1997) Brain MR imaging in patients with hepatic cirrhosis: relationship between high intensity signal in basal ganglia on T1-weighted images and elemental concentrations in brain. Neuroradiology 39:546–550
Mochizuki H, Kamakura K, Masaki T et al (1997) Atypical MRI features of Wilson’s disease: high signal in globus pallidus on T1-weighted images. Neuroradiology 39:171–174
Herynek V, Babis M, Trunecka P et al (2001) Chronic liver disease: relaxometry in the brain after liver transplantation. MAGMA 12:10–15
Prosch H, Grois N, Wnorowski M, Steiner M, Prayer D (2007) Long-term MR imaging course of neurodegenerative Langerhans cell histiocytosis. AJNR Am J Neuroradiol 28:1022–1028
Herynek V, Wagnerová D, Malucelli A, Vymazal J, Sameš M, Hájek M (2015) Alterations in the basal ganglia in patients with brain tumours may be due to excessive iron deposition. Oncol Lett 9:43–46
McDonald RJ, McDonald JS, Kallmes DF et al (2015) Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 275:772–782
Kanda T, Fukusato T, Matsuda M et al (2015) Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 276:228–232
Murata N, Gonzalez-Cuyar LF, Murata K et al (2016) Macrocyclic and other non-group 1 gadolinium contrast agents deposit low levels of gadolinium in brain and bone tissue: preliminary results from 9 patients with normal renal function. Invest Radiol 51:447–453
Agris J, Pietsch H, Balzer T (2016) What evidence is there that gadobutrol causes increasing signal intensity within the dentate nucleus and globus pallidus on unenhanced T1 W MRI in patients with RRMS? Eur Radiol 26:816–817
Rossi Espagnet M, Tomà P, Napolitano A (2017) Reply to Lancelot et al.: ‘lack of evidence of a relationship between magnetic resonance signal intensity changes in the globus pallidus and dentate nucleus, and repeated administrations of gadoterate meglumine in children. Pediatr Radiol. doi:10.1007/s00247-017-3948-0
Rossi Espagnet M, Tomà P, Napolitano A (2017) Reply to Radbruch et al.: ‘interpreting signal-intensity ratios without visible T1 hyperintensities in clinical gadolinium retention studies’ Pediatr Radiol (2017). Pediatr Radiol. doi:10.1007/s00247-017-3971-1
Tanaka M, Nakahara K, Kinoshita M (2016) Increased signal intensity in the dentate nucleus of patients with multiple sclerosis in comparison with neuromyelitis optica spectrum disorder after multiple doses of gadolinium contrast. Eur Neurol 75:195–198
Schlemm L, Chien C, Bellmann-Strobl J et al (2017) Gadopentetate but not gadobutrol accumulates in the dentate nucleus of multiple sclerosis patients. Mult Scler 23:963–972
Eisele P, Alonso A, Szabo K et al (2016) Lack of increased signal intensity in the dentate nucleus after repeated administration of a macrocyclic contrast agent in multiple sclerosis: an observational study. Medicine (Baltimore) 95:e4624
Eisele P, Szabo K, Alonso A et al (2017) Lack of T1 hyperintensity in the dentate nucleus after 15 administrations of a macrocyclic contrast agent in multiple sclerosis. J Neurol Neurosurg Psychiatry. [Epub ahead of print]
Patriarca L, Torlone S, Ferrari F, Di Carmine C, Totaro R, Di Cesare E, Splendiani A (2016) Is size an essential criterion to define tumefactive plaque? MR features and clinical correlation in multiple sclerosis. Neuroradiol J 29:384–389
Totaro R, Di Carmine C, Splendiani A, Torlone S, Patriarca L, Carrocci C, Sciamanna S, Marini C, Carolei A (2016) Occurrence and long-term outcome of tumefactive demyelinating lesions in multiple sclerosis. Neurol Sci 37:1113–1117
Splendiani A, Felli V, Di Sibio A, Gennarelli A, Patriarca L, Stratta P, Di Cesare E, Rossi A, Massimo G (2016) Magnetic resonance imaging and magnetic resonance spectroscopy in a young male patient with anti-N-methyl-D-aspartate receptor encephalitis and uncommon cerebellar involvement: a case report with review of the literature. Neuroradiol J 29:30–35
Marsecano C, Perri M, Michelini G, Varrassi M, Splendiani A, Di Cesare E, Masciocchi C, Gallucci M (2015) Vascular malformation mimicking multiple sclerosis active plaque: usefulness of susceptibility weighted imaging (SWI) to perform correct diagnosis. Neuroradiol J 28:488–492
Splendiani A, Mariani S, Anselmi M, Catalucci A, Di Cesare E, Gallucci M (2015) Neuromyelitis optica: atypical clinical and neuroradiological presentation. Neuroradiol J 28:42–45
Felli V, Di Sibio A, Anselmi M, Gennarelli A, Sucapane P, Splendiani A, Catalucci A, Marini C, Gallucci M (2014) Progressive multifocal leukoencephalopathy following treatment with rituximab in an HIV-negative patient with non-hodgkin lymphoma: a case report and literature review. Neuroradiol J 27:657–664
Splendiani A, Catalucci A, Limbucci N, Turner M, Krings T, Gallucci M (2012) Pediatric inflammatory diseases. Part III: small vessels vasculitis. Neuroradiol J 25:715–724
Catalucci A, Anselmi M, Splendiani A, Smith JD, Limbucci N, Giangaspero F, Gallucci M (2012) Pediatric inflammatory diseases. Part I: multiple sclerosis. Neuroradiol J 25:684–694
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612
Lancelot E (2016) Revisiting the pharmacokinetic profiles of gadolinium-based contrast agents: differences in long-term biodistribution and excretion. Invest Radiol 51:691–700
Diedrichsen J, Maderwald S, Küper M et al (2011) Imaging the deep cerebellar nuclei: a probabilistic atlas and normalization procedure. Neuroimage 54:1786–1794
Grobner T (2006) Gadolinium–a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant 21:1104–1108 (Epub 2006 Jan 23. No abstract available. Erratum in: Nephrol Dial Transplant. 2006; 21:1745)
Thomsen HS, Morcos SK, Almén T et al (2013) Nephrogenic systemic fibrosis and gadolinium-based contrast media: updated ESUR Contrast Medium Safety Committee guidelines. Eur Radiol 23:307–318
Heverhagen JT, Krombach GA, Gizewski E (2014) Application of extracellular gadolinium-based MRI contrast agents and the risk of nephrogenic systemic fibrosis. Rofo 186:661–669
Lauenstein T, Ramirez-Garrido F, Kim YH et al (2015) Nephrogenic systemic fibrosis risk after liver magnetic resonance imaging with gadoxetate disodium in patients with moderate to severe renal impairment: results of a prospective, open-label, multicenter study. Invest Radiol 50:416–422
Martin DR, Kalb B, Mittal A, Salman K, Vedantham S, Mittal PK (2017) No incidence of nephrogenic systemic fibrosis after gadobenate dimeglumine administration in patients undergoing dialysis or those with severe chronic kidney disease. Radiology 21:170102 (Epub ahead of print)
Endrikat J, Vogtlaender K, Dohanish S, Balzer T, Breuer J (2016) Safety of gadobutrol: results from 42 clinical phase II to IV studies and postmarketing surveillance after 29 million applications. Invest Radiol 51:537–543
Lee H, Mortensen K, Sanggaard S et al (2017) Quantitative Gd-DOTA uptake from cerebrospinal fluid into rat brain using 3D VFA-SPGR at 9.4T. Magn Reson Med. doi:10.1002/mrm.26779 (Epub ahead of print)
Enzmann DR, Pelc NJ (1991) Normal flow patterns of intracranial and spinal cerebrospinal fluid defined with phase-contrast cine MR imaging. Radiology 178:467–474
Greenberg SA (2010) Zinc transmetallation and gadolinium retention after MR imaging: case report. Radiology 257:670–673
Maximova N, Gregori M, Zennaro F, Sonzogni A, Simeone R, Zanon D (2016) Hepatic gadolinium deposition and reversibility after contrast agent-enhanced MR imaging of pediatric hematopoietic stem cell transplant recipients. Radiology 281:418–426
Tweedle MF, Hagan JJ, Kumar K, Mantha S, Chang CA (1991) Reaction of gadolinium chelates with endogenously available ions. Magn Reson Imaging 9:409–415
Bussi S, Coppo A, Botteron C et al (2017) Differences in gadolinium retention after repeated injections of macrocyclic MR contrast agents to rats. J Magn Reson Imaging. doi:10.1002/jmri.25822 (Epub ahead of print)
Tedeschi E, Palma G, Canna A et al (2016) In vivo dentate nucleus MRI relaxometry correlates with previous administration of Gadolinium-based contrast agents. Eur Radiol 26:4577–4584
Roccatagliata L, Vuolo L, Bonzano L, Pichiecchio A, Mancardi GL (2009) Multiple sclerosis: hyperintense dentate nucleus on unenhanced T1-weighted MR images is associated with the secondary progressive subtype. Radiology 251:503–510
Absinta M, Rocca MA, Filippi M (2011) Dentate nucleus T1 hyperintensity in multiple sclerosis. AJNR Am J Neuroradiol 32:E120–E121
Author information
Authors and Affiliations
Contributions
Dr. Splendiani is the principal investigator. Other authors have equally contributed in drafting/revising the manuscript for content, including medical writing for content, study concept or design, and analysis or interpretation of data.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards and patient consent
We declare that all human and studies have been approved by the local ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Rights and permissions
About this article
Cite this article
Splendiani, A., Perri, M., Marsecano, C. et al. Effects of serial macrocyclic-based contrast materials gadoterate meglumine and gadobutrol administrations on gadolinium-related dentate nuclei signal increases in unenhanced T1-weighted brain: a retrospective study in 158 multiple sclerosis (MS) patients. Radiol med 123, 125–134 (2018). https://doi.org/10.1007/s11547-017-0816-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-017-0816-9